To utilize low-cost and simple methods to assess airway and lung inflammation biomarkers related to air pollution.
METHODS:A total of 87 male, non-smoking, healthy subjects working as street traffic-controllers or office-workers were examined to determine carbon monoxide in exhaled breath and to measure the pH in nasal lavage fluid and exhaled breath condensate. Air pollution exposure was measured by particulate matter concentration, and data were obtained from fixed monitoring stations (8-h work intervals per day, during the 5 consecutive days prior to the study).
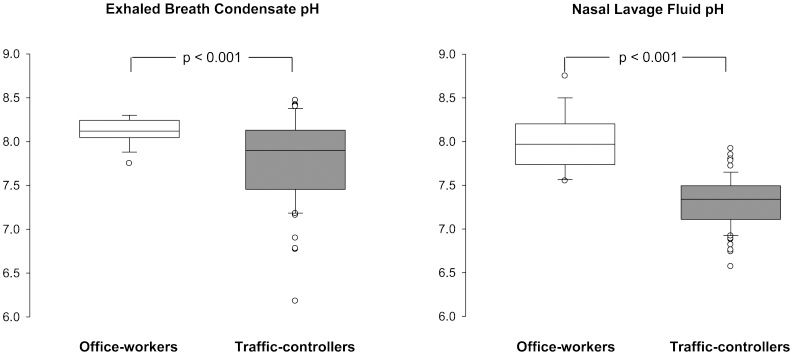
RESULTS:Exhaled carbon monoxide was two-fold greater in traffic-controllers than in office-workers. The mean pH values were 8.12 in exhaled breath condensate and 7.99 in nasal lavage fluid in office-workers; these values were lower in traffic-controllers (7.80 and 7.30, respectively). Both groups presented similar cytokines concentrations in both substrates, however, IL-1β and IL-8 were elevated in nasal lavage fluid compared with exhaled breath condensate. The particulate matter concentration was greater at the workplace of traffic-controllers compared with that of office-workers.
CONCLUSION:The pH values of nasal lavage fluid and exhaled breath condensate are important, robust, easy to measure and reproducible biomarkers that can be used to monitor occupational exposure to air pollution. Additionally, traffic-controllers are at an increased risk of airway and lung inflammation during their occupational activities compared with office-workers.
There is epidemiological evidence that air pollution is directly associated with increases in respiratory symptoms, pulmonary inflammation, infections, emergency room visits and hospital admissions (1–5). Among the pathophysiological mechanisms of these events, in vitro and in vivo studies have shown that air pollutants, particularly fine and ultra-fine particles, induce the release of reactive oxygen species into airways and cause lung inflammation (6–8).
In São Paulo, which is one of the largest cities in the world, seven million vehicles circulate daily, resulting in traffic jam chaos in several areas of the city. Because of the high levels of vehicle-released pollutants in the atmosphere, outdoor activities in these areas may pose an increased health hazard, particularly to the respiratory system. Inflammation in the airways and lungs has an important role in the development and progression of several respiratory diseases.
We examined the air pollution-related airway and lung inflammation in non-smoking, healthy street traffic-controllers and office-workers by measuring the pH and cytokines concentrations in exhaled breath condensate (EBC) and nasal lavage fluid (NLF) using low-cost and simple methods (9–16). Both EBC and NLF contain particles from the upper and the lower airway lining fluid and may be potential sources of air pollution-related inflammatory biomarkers.
MATERIALS AND METHODSStudy populationThis cross-sectional study was approved by the local Ethics Committee of Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (CAPPesq 0565/07). Non-smoking healthy male subjects aged 18 to 60 years were recruited from a list provided by the Engineering Traffic Company of São Paulo City (street traffic-controllers) and from Faculdade de Medicina da Universidade de São Paulo (office-workers). The subjects were enrolled in the study after providing informed consent. The exclusion criteria were as follows: the use of any chronic medications or the presence of any diagnosed acute or chronic disease. A healthy status was defined after a medical examination of each subject.
Study designThe subjects were assessed only once after one work week (five days of 8-h shifts) at the University Laboratory of Pulmonary Defense on a Saturday morning (from 8 A.M. until noon). The subjects were asked to sit in a chair in a quiet room. Clinical and job history, arterial blood pressure (mmHg), heart rate (bpm), pulse oximetry (%) and respiratory rate (rpm) were registered for each subject after 10 min of rest. The other variables were determined as described below.
Exhaled carbon monoxide measurementsThe concentrations of exhaled carbon monoxide (CO) were determined (in ppm) with the aid of a Micro CO analyzer (Cardinal Health U.K., 232 Ltd., Chatham, UK). The subjects were asked to exhale slowly from their total lung capacity with a constant expiratory flow of 5–6 l min−1 over 10 to 15 sec. The mean of two reproducible measurements with a variation of less than 5% was considered.
Exhaled breath condensateThe EBC was obtained as previously described (10). At the start of EBC collection, all subjects rinsed their mouths with distilled water and were instructed to swallow saliva as necessary and to hold a slight head extension (approximately 15°). The EBC sample was collected over 15 min of quiet and normal breathing (regular tidal volumes and respiratory rate) through a mouthpiece that was connected to a collector device with dry ice (−20°C). The total EBC (2.0–2.5 ml) was immediately divided and transferred to sterile 500 μl polypropylene tubes. One aliquot was immediately used for pH measurements. The remaining EBC sample aliquots were coded (for blinding purposes) and stored for a maximum of 4 weeks at −80°C for the determination of cytokine levels.
Nasal lavage collectionSubjects were asked to tilt their head back at a 45° angle and close the nasopharynx with the soft palate. Room temperature isotonic sodium chloride solution (0.9% NaCl, 5 ml) was instilled into each nostril. After 10 sec, the subject blew their nose forcefully into a sterile plastic container. The average recovery of fluid from NLF was approximately 70%. The lavage fluid was centrifuged (10 min, 300 g, 5°C), and the supernatant was separated from the pellet and divided into five aliquots of 500 μl. One of these supernatant aliquots was immediately used for pH measurements. The remaining aliquots were coded (for blinding purposes) and stored at −80°C for up to 4 weeks to determine the cytokine levels. The cell pellet was used for total and differential cell counts as previously described (15,16).
Total and differential cell counts in the NLFThe cell pellet was resuspended in 1 ml of phosphate buffer saline solution (PBS). Thereafter, 20 μl of the mixed solution was added to a Neubauer chamber, and the cells were counted using a 400x light microscope (Olympus CH2, Olympus America Inc., Palo Alto, USA). For differential cell proportions (%), 100 μl of the mixed solution was centrifuged (96 g, 25°C, 6 min) to obtain a slide with two areas of cells that were stained with hematoxylin and eosin. Differential cell counts were performed by two different observers with the aid of a 1000x light microscope (Olympus CH2, Olympus America Inc., Palo Alto, USA) (15,16).
pH measurements in EBC and NLFIn a room maintained at a constant ambient temperature (23°C) and relative humidity (65%), 500 μl of fluid (EBC or NLF) was de-aerated with a gentle 350 ml/min flow of ultrapure (99.9%) argon gas (Gama Gases Ltd., Sao Paulo, Brazil) for 15 min. The pH was determined with the aid of a microelectrode and a pH meter (827 pH Lab, Metrohm Ltd., Herisau, Switzerland). The pH meter was calibrated before each measurement using solutions with pH values of 4, 7 and 9. After pH determination, the EBC and NLF aliquots were discarded (9–11).
Measurement of cytokines in EBC and NLFThe concentrations of tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-8 and IL-10 in EBC and NLF samples were determined using high sensitivity enzyme-immunoassays (Quantikine HS, R&D Systems Inc., Minneapolis, USA). The limits of detection of the assays were as follows: (a) TNF-α: 0.106 pg/ml, with the standard curve fitted between 0 and 32 pg/ml; (b) IL-1β: 0.057 pg/ml, with the standard curve fitted between 0 and 8 pg/ml; (c) IL-8: 3.50 pg/ml, with the standard curve fitted between 0 and 2,000 pg/ml; and (d) IL-10: 0.50 pg/ml, with the standard curve fitted between 0 and 50 pg/ml.
Air pollutantsThe estimation of the exposure of each volunteer (traffic-controller or office-worker) to 10 μm diameter particulate matter (PM10) was obtained during five consecutive days of an 8-h work shift from one of the seven fixed monitoring stations of the São Paulo State Environmental Agency in São Paulo City (Ibirapuera, Pinheiros, Cerqueira Cesar, Centro, Congonhas, Mooca and Parque Dom Pedro). The fixed monitoring stations that were geographically closest to the workplaces of the volunteers were chosen. None of the workplaces was >5 km distant from a fixed monitoring station.
Statistical analysisNormality distribution was assessed using normal probability plots. The data are expressed as the mean and standard deviation (SD) when normally distributed or the median and interquartile range (IQR) when otherwise appropriate. Comparisons between groups (traffic-controllers and office-workers) were performed using the t-test or Mann-Whitney test. Pearson's correlation or Spearman's rank correlation coefficients were utilized to quantify the degree of association between variables. The estimated effect of traffic-controllers on the pH and the comparison between substrates (EBC and NLF) was determined using linear multiple regression adjusted for age and body mass index (BMI). Interactions between substrate and group and substrate and age were included in the model. The amount of variability in the response variable explained by the model was evaluated by the coefficient of determination, R2. To compare cytokines distributions between groups and between substrates, we created a factor with 4 levels (the combination between groups and substrates), and we used the Kruskal-Wallis test followed by Bonferroni's method (when necessary) to localize the differences. Statistical analyses were carried out using the SPSS statistical package, version 15 (SPSS Inc., Chicago, IL, USA). No correction for the multiplicity of tests was performed; however, p-values are given explicitly wherever reasonable. Statistical significance was set at 5%.
RESULTSThe demographic and clinical characteristics of the 87 adult male subjects who entered into the study (Table 1) showed differences in age and BMI between the street traffic-controllers and office-workers. However, the two occupational groups had similar vital signs (arterial blood pressure, heart rate, pulse oximetry and respiratory rate). Despite being within the normal range, the median levels of exhaled CO were two-fold higher in the street traffic-controllers compared with the office-workers.
Demographic and clinical data of the street traffic-controllers and office-workers.
| Traffic-controllers n = 73 | Office-workers n = 14 | p-value | |
|---|---|---|---|
| Age, years, mean (SD) | 42 (7) | 30 (5) | <0.001* |
| Body mass index, kg/m2, mean (SD) | 27.4 (3.7) | 24.5 (3.0) | 0.005* |
| Systolic blood pressure, mmHg, mean (SD) | 118 (13) | 118 (12) | 0.923* |
| Diastolic blood pressure, mmHg, mean (SD) | 82 (8) | 78 (11) | 0.261* |
| Heart rate, bpm, mean (SD) | 69 (9) | 70 (10) | 0.792* |
| Respiratory rate, rpm, mean (SD) | 15 (3) | 14 (2) | 0.186* |
| Exhaled CO, ppm, median (IQR) | 4.5 (3) | 2.5 (1.3) | <0.001† |
Abbreviations: CO, carbon monoxide; *, t test; †, Mann-Whitney test.
The correlation between pH in the EBC and in the NLF was r = 0.12 and p = 0.38 in the traffic-controllers and r = −0.31 and p = 0.277 in the office-workers. The EBC pH was not correlated with age (r = 0.07 and p = 0.496); however, it was correlated with BMI (r = 0.22 and p = 0.039). The NLF pH was correlated with both age (r = −0.60 and p<0.001) and BMI (r = −0.27 and p = 0.011).
The pH measurements indicated that the EBC and NLF of traffic-controllers were more acidic than the EBC and NLF of office-workers (Figure 1). In the multiple linear regression model, there was a mean reduction in pH of −0.42 (SE = 0.13, p = 0.001), which was independent of the substrate (EBC or NLF, p = 0.608).
Cytokine expression in EBC and NLFThe concentrations of cytokines between EBC and NLF were not correlated. No significant differences in IL-10 were observed between the street traffic-controllers and the office-workers and between the substrates (EBC and NLF) (p = 0.455) (Table 2). However, significantly greater concentrations of IL-1 and IL-8 were observed in the NLF of traffic-controllers and office-workers compared with the EBC.
Cytokines concentrations in the exhaled breath condensate and nasal lavage fluid of the traffic-controllers and office-workers. The data were analyzed by the Kruskal-Wallis test and Bonferroni post-hoc test. The results are presented as median values (IQRs).
| Group | EBC | NLF | p-value | |
|---|---|---|---|---|
| TNF-α (pg/ml) | Traffic-controllers | 0.5 (0.3) | 0.4 (0.4) | 0.590 |
| Office-workers | 0.4 (0.0) | 0.3 (0.2) | 0.879 | |
| IL-1β (pg/ml) | Traffic-controllers | 0 (0.7) | 8.4 (5.2) | <0.001 |
| Office-workers | 0 (0.0) | 8.4 (5.4) | <0.001 | |
| IL-8 (pg/ml) | Traffic-controllers | 8.9 (1.8) | 257.0 (411.2) | <0.001 |
| Office-workers | 8.4 (0.9) | 293.6 (589.6) | <0.001 | |
| IL-10 (pg/ml) | Traffic-controllers | 1.2 (1.6) | 1.2 (2.7) | 0.455 |
| Office-workers | 1.0 (1.4) | 1.5 (2.2) | 0.455 |
TNF-α, tumor necrosis factor-alpha; IL-1β, interleukin-1 beta; IL-8, interleukin-8; IL-10, interleukin-10.
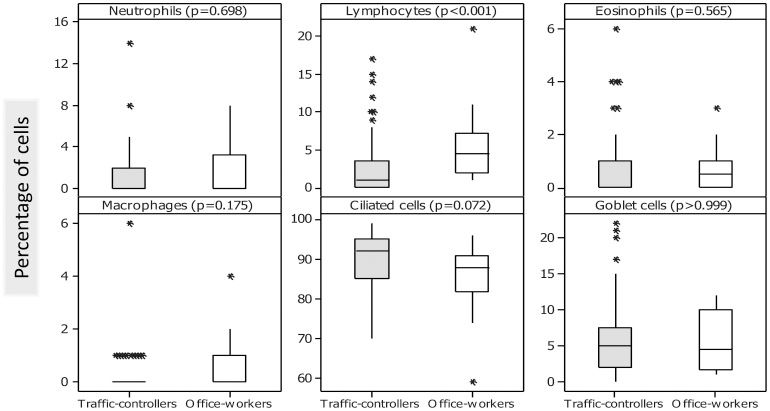
The number of total cells (median and IQR) in the NLF of the office-workers (126 and 170 cells/mm3) was greater than that of the traffic-controllers (42 and 77 cells/mm3), with an increase in lymphocytes (Figure 2). However, no other significant differences were observed in the NLF of office-workers and traffic-controllers with respect to the percentages of neutrophils, eosinophils, macrophages and epithelial cells (ciliated and goblet cells).
Environmental exposure dataThe outdoor mean PM10 concentrations (SD) in the workplaces of the street traffic-controllers and office-workers were 26.4 (9.45) μg/m3 and 19.7 (1.71) μg/m3, respectively, and were significantly different (p = 0.006). No significant correlations were found between the PM10 concentrations and the EBC pH (r = −0.19 and p = 0.125), the NLF pH (r = −0.21 and p = 0.07), as either with exhaled CO (r = 0.21 and p = 0.876). However, among the office-workers, the correlation coefficient between the NLF pH and PM10 was r = −0.46 (p = 0.154) as a result of the lower statistical power due to the reduced PM10 sample size (n = 11).
DISCUSSIONWe conducted a cross-sectional study to assess the effects of two levels of air pollution exposure on human airways: a busy street with considerable traffic and the inside of an office. Because street traffic-controllers perform their occupational activities under direct exposure to outdoor vehicle-related air pollution, they are at increased risk of airway inflammation. Street traffic-controllers presented lower NLF and EBC pH values accompanied by increased amounts of exhaled CO compared with office-workers. However, the concentrations of IL-1β and IL-8 in NLF and EBC were similar in traffic-controllers and office-workers. The workplace of the street traffic-controllers was 25% more polluted than the workplace of the office-workers, although the PM10 concentrations were within the air quality limits advocated by international environmental agencies.
Inflammation is a physiological response to a variety of stimuli comprising a complex series of events that involve macrophages, monocytes and neutrophils among others and their molecular products (i.e., cytokines and chemokines). Exposure to air pollution may pose a significant health risk to some outdoor professionals. The nasal mucosa is the first barrier of the respiratory system that protects against inhaled pollutants and other agents and plays an important role in the innate immune response to environmental stimuli (17). This response is often studied using nasal biopsies, which themselves induce inflammatory responses. We have used low-cost and simple methods to study the response of the nasal mucosa and lower airways; these methods involve the collection of NLF and EBC. In the present study, we obtained sufficient amounts of sampling fluid, and both were effective sources of inflammatory biomarkers that provided complementary information. The acidification of the EBC suggests endogenous airway inflammation, which is implicated in the pathophysiology of several respiratory disorders. All the volunteers in this study were healthy non-smoking adults with no history of acute or chronic respiratory disease. The office-workers had a mean EBC pH of 8.12, which is within the normal values reported by others (10,18–21). However, the street traffic-controllers showed significant reductions in the EBC pH values. The lower pH level was similar to that observed in mechanically ventilated patients experiencing respiratory failure in the intensive care unit (18).
Experimental and human studies have shown that exposure to air pollutants may generate reactive oxygen species due to the presence of free radicals and oxidants on the particle surface (22) and may cause alterations in the transcription of inflammatory cytokines (7). Because cytokines may play important roles in the pathophysiology of airway and lung parenchyma diseases, interest has been focused on cytokines determinations (23). IL-1β plays an important protective role against pathogen invasion because it is a potent inflammatory mediator that stimulates chemokine production and leukocyte recruitment to the site of injury. Additionally, IL-1β is involved in a variety of cellular activities including cell proliferation, differentiation and apoptosis (24). IL-8 is responsible for the recruitment of inflammatory cells from the circulation to the airways in many respiratory diseases (25,26). Few studies on health effects related to air pollution have explored cytokines levels in EBC, primarily because low concentrations of cytokines have been reported (27,28). Indeed, in the present study, the IL-1β concentrations in EBC were between 0 and 1 pg/ml, similar to the findings of other reports in healthy subjects (27,29). In this study, the IL-8 concentrations in EBC were similar in the office-workers and the street traffic-controllers (∼10 pg/ml); however, these concentrations were four- to nine-fold higher than previous findings in healthy individuals (15,30,31) and similar to those observed young smokers (30,32). We can not exclude the possibility that these differences can be explained by the use of different standards in the assays.
In NLF, we found an increased number of total cells in office-workers, which was primarily due to an increased number of lymphocytes. In contrast, traffic-workers presented a similar trend in the number of ciliated cells, although statistical significance was not achieved. We also found higher concentrations of IL-1β and IL-8 in NLF compared with EBC (eight-fold and three-fold, respectively). Data related to IL-1β levels in NLF are very scarce in the literature. Riechelmann et al. (25) reported IL-1β concentrations of 15±13 pg/ml in healthy non-smoking volunteers (aged 18-60 years); in the current study, we observed IL-1β concentrations that were 50% lower. However, the IL-8 concentrations in the NLF samples in the present study were ten-fold greater than those in volunteers with occupational rhinitis (mean IL-8 concentrations of ∼27 pg/ml) (33). In agreement with this finding, other reports have shown direct associations between exposure to PM2,5 and nasal inflammation (34) as well as increases in IL-8 production in the airways of healthy individuals after acute exposure to diesel exhaust (35). In the present study, the levels of IL-1β and IL-8 were increased in NLF compared with EBC in the traffic-controllers and office-workers, and these increases were not accompanied by changes in the percentage of neutrophils. This finding raised the possibility that the increase in these pro-inflammatory mediators (IL-1β and IL-8) may have resulted from epithelial cell production, as reported in in vitro studies (36) with human bronchial epithelial cells exposed to PM10 and in studies of the nasal epithelial cells of asthmatic children (37).
Human activity patterns and microenvironmental exposure can significantly affect exhaled CO levels; however, it has been suggested that regular monitoring of exhaled CO levels in healthy subjects has the potential to be used as a functional index of air pollution (38). We observed higher exhaled CO levels in traffic-controllers (two-fold) than in office-workers, although both levels were within the normal values for non-smokers (<10 ppm, according to the ATS guidelines).
This study has limitations. There were some differences in demographic characteristics between our study groups. For example, the street traffic-controllers were older than the office-workers. However, previous studies have shown that aging has no effect on the pH of EBC (18), with the exception of individuals between 60 and 80 years old (20), which was not the age range of our study population. Additionally, the BMI of the traffic-controllers was higher than that of the office-workers. Obesity has been reported to be associated with systemic inflammation, particularly if coupled with sedentary behavior (39). However, the difference in BMI between the two groups was only approximately 10; thus, it may not have affected our results. In the present study, the outdoor air pollution data must be interpreted with caution. We used the PM10 concentration data provided by fixed monitoring stations that were located near the workplace of each street traffic-controller and office-worker (mean values of 8-hr intervals over 5 consecutive work days). We detected a 25% difference in exposure between the traffic-controllers and the office-workers. However, this difference may still underestimate the PM10 exposure among traffic-controllers because considerably higher levels of pollutants are present at the location where the traffic-controllers work compared with the city average, as we have recently demonstrated in vehicle corridors with high traffic (40). In addition, the PM10 results likely do not reflect the real difference in personal exposure (41). However, unpublished data from our laboratory show that the mean PM10 concentration measured by fixed monitoring stations is 50% lower than the personal exposure. Another issue is that the personal exposure of office-workers may be lower than the value reported by the fixed monitoring stations because these individuals work indoors. Several reports indicate that exposure to urban traffic is markedly attenuated in the indoor environment (41). Additionally, the present study was performed during a period of school vacation when there were 30–40% fewer vehicles circulating in the city. However, we found that the pH in NLF and EBC was an efficient biomarker that can be used to assess the inflammatory effects of air pollution on the airways and lungs. Inflammatory disturbances were markedly present in the nasal cavities of both groups. However, only traffic-controllers showed extended inflammation in the airways based on acidification of their EBC.
In conclusion, the pH values of NLF and EBC are important, robust, easy-to-measure and reproducible biomarkers that can be used to monitor occupational exposure to air pollution. Additionally, traffic-controllers are at an increased risk of airway and lung inflammation during their occupational activities compared with office-workers.
ACKNOWLEDGMENTSThe authors would like to thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 07/51605-9 and 09/50056-7) and the National Council of Technological and Scientific Development (CNPq 555.223/06-0) for providing financial support for this study. We also thank Carolina Tieko Yoshida for revising tables, figures and references.
All the authors participated in the study design, results analysis, discussion and manuscript writing. Lima TM, Kazama CM, Santos UP, Bueno-Garcia ML and Nakagawa NK also participated in the data collection.
No potential conflict of interest was reported.