We explored whether high blood pressure is associated with metabolic, inflammatory and prothrombotic dysregulation in patients with metabolic syndrome.
METHODS:We evaluated 135 consecutive overweight/obese patients. From this group, we selected 75 patients who were not under the regular use of medications for metabolic syndrome as defined by the current Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults criteria. The patients were divided into metabolic syndrome with and without high blood pressure criteria (≥130/≥85 mmHg).
RESULTS:Compared to the 45 metabolic syndrome patients without high blood pressure, the 30 patients with metabolic syndrome and high blood pressure had significantly higher glucose, insulin, homeostasis model assessment insulin resistance index, total cholesterol, low-density lipoprotein-cholesterol, triglycerides, uric acid and creatinine values; in contrast, these patients had significantly lower high-density lipoprotein-cholesterol values. Metabolic syndrome patients with high blood pressure also had significantly higher levels of retinol-binding protein 4, plasminogen activator inhibitor 1, interleukin 6 and monocyte chemoattractant protein 1 and lower levels of adiponectin. Moreover, patients with metabolic syndrome and high blood pressure had increased surrogate markers of sympathetic activity and decreased baroreflex sensitivity. Logistic regression analysis showed that high-density lipoprotein, retinol-binding protein 4 and plasminogen activator inhibitor-1 levels were independently associated with metabolic syndrome patients with high blood pressure. There is a strong trend for an independent association between metabolic syndrome patients with high blood pressure and glucose levels.
CONCLUSIONS:High blood pressure, which may be related to the autonomic dysfunction, is associated with metabolic, inflammatory and prothrombotic dysregulation in patients with metabolic syndrome.
In 1988, the cardiovascular risk factor cluster, which includes obesity, increased blood pressure, high triglycerides and glucose and low HDL (high-density lipoprotein)-cholesterol, was given the name “metabolic syndrome” (MetS) (1). Since then, the scientific community has tried to better define whether MetS predicts cardiovascular morbidity and mortality better than the sum of the individual components and whether one component is pivotal over the others. Most evidence points to visceral obesity and insulin resistance as central features of MetS. Little attention has been given to exploring other components of MetS, such as blood pressure and the potential factors that can influence not only blood pressure but also the metabolic dysregulation observed in MetS.
Obesity-related sympathetic activation is an attractive explanation for several components of MetS. In particular, in 1994, the aggregation of cardiovascular risk factors and signs of a hyper sympathetic state was demonstrated in the Tecumseh population (2). Indeed, increased sympathetic activity (faster heart rate, higher cardiac output and plasma noradrenaline) correlated with higher levels of glucose, insulin, cholesterol, triglycerides, body weight and hematocrit and lower levels of HDL-cholesterol. In the Framingham Heart Study (3), more than 50% of hypertensive patients had 2 or more metabolic abnormalities. Only 19% of males and 17% of females had isolated hypertension. In view of this finding, the name hypertensive syndrome had been used in the past (4). However, it is not clear whether the increase in blood pressure is associated with exacerbations of the metabolic, proinflammatory, prothrombotic, vascular and autonomic dysfunctions in patients with MetS.
Evidence suggests that autonomic dysregulation also contributes to elevated blood pressure and metabolic abnormalities. Moreover, α1-adrenoceptor antagonists lower blood pressure, improve insulin sensitivity and ameliorate dyslipidemia (5). However, it is unclear whether autonomic dysregulation in metabolic syndrome is associated not only with hemodynamic impairment but also with metabolic, inflammatory and other abnormalities associated with the syndrome. We hypothesized that high blood pressure, which may reflect increased sympathetic activity, is independently associated with metabolic dysregulation and inflammation in patients with MetS.
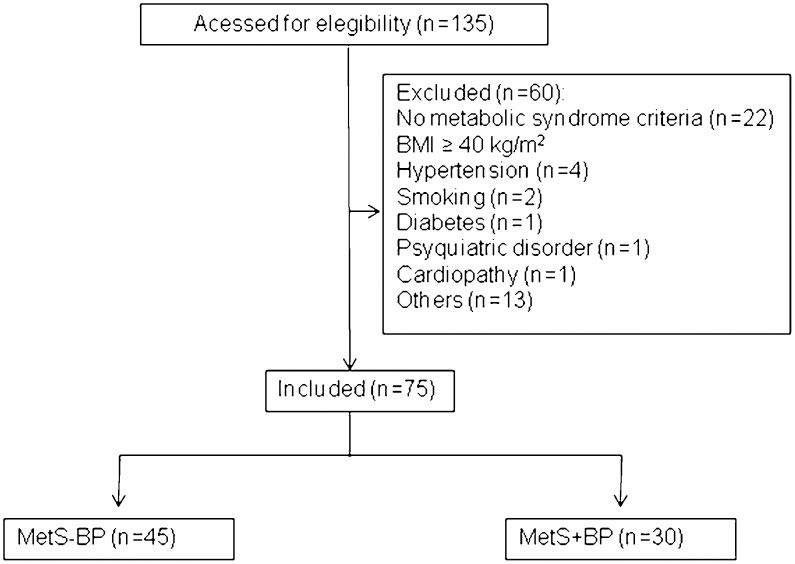
METHODSStudy populationThis research was performed at the Heart Institute (InCor) at the University of São Paulo Medical School. Over a 1-year period, we initially evaluated 135 consecutive overweight or obese patients from the São Paulo metropolitan area. From this group, we selected 75 patients with MetS, diagnosed according to ATP III (Third Report of the Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults criteria, and with at least three of the following criteria: high blood pressure - arterial blood pressure ≥130 and/or ≥85 mmHg for systolic and diastolic blood pressure, respectively; high glucose - fasting glucose ≥100 mg/dL; increased waist circumference - ≥102 cm in men and ≥88 in women; increased triglycerides - ≥150 mg/dL; decreased HDL - <40 mg/dL in men and <50 mg/mg/dL in women) (6). We excluded patients with morbid obesity (body mass index ≥40 kg/m2), severe hypertension (BP >180/110 mmHg), under regular physical activity, secondary forms of hypertension, diabetes, smokers, patients with any chronic disease and regularly using medications (including antihypertensives, see Figure 1). The patients were divided into 2 groups according to blood pressure status (patients with MetS without high blood pressure, i.e., <130/<85 mmHg: MetS-BP) and (patients with MetS with high blood pressure, i.e., ≥130 systolic and/or ≥85 mmHg diastolic: MetS+BP).
Clinical evaluationPatients who fulfilled the criteria for the study underwent a medical history evaluation. Blood pressure was measured in triplicate after 5 minutes of seated rest using a calibrated sphygmomanometer by the same investigator (J.S.G). The average from 3 blood pressure values was used to define the presence or absence of the ATP III, the criterion used to define elevated blood pressure (≥130 or ≥85 mmHg for systolic and diastolic blood pressure levels, respectively). Fasting blood samples were drawn for biochemical tests and cytokine levels. Serum and plasma were obtained and then stored at -80°C. During the second visit, anthropometric measurements (body mass index – BMI; waist and hip circumferences; and bicipital, tricipital, subscapular and suprailiac skinfolds) and fat mass, lean mass, basal metabolism and total body water were measured using bioimpedance-measuring equipment (BIA 450, Bio dynamics, Seattle, USA) (7).
Biochemistry measurementsTotal cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, uric acid, glucose and creatinine levels were measured using commercial kits.
Insulin resistanceInsulin resistance was measured using the following 2 methods: the traditional Homeostasis Model Assessment Insulin Resistance method (HOMA-IR) and the Retinol-Binding Protein 4 method (RBP4). The HOMA-IR index was calculated as [fasting serum glucose (mmol/L) x fasting serum insulin (UI/mL)]/22.5.
CytokinesCytokines, including total adiponectin, resistin, leptin, insulin, interleukin 6 (IL-6), tumoral necrosis factor alpha (TNF-α), plasminogen activator inhibitor 1 (PAI-1) and monocyte chemoattractant protein 1 (MCP-1), were measured using a multiplex sandwich enzyme-linked immunosorbent assay (ELISA, Millipore, MA, USA) (8).
Power spectral analysisThe data for spectral analysis were derived from a finger pulse contour recorded by beat-to-beat blood pressure measurements (Finometer®, FMS, Finapres Medical System BV, Holland) and from the electrocardiogram (ECG) for 10 minutes to characterize the RR interval series. Power spectral density was obtained by the fast Fourier transformation, using Welch's method with a Hanning window of 512 points and 50% overlap as previously described (9). The entire time, series were previously re-sampled at 5.0 Hz to be equally spaced in time by a special signal editor developed for Matlab (MATLAB 6.0; Mathworks, Natick, Massachusetts, USA). Interpolated time series were decimated to be equally time-spaced. Two main components were considered for the RR interval variability: low frequency (LF; 0.04 to 0.15 Hz) and high frequency (HF; 0.15 to 0.4 Hz). The power density of each spectral component from the RR interval was calculated in absolute values and normalized units. The normalized units were obtained by calculating the percentages of LF variability (LFms2) and HF variability (HFms2) with respect to the total power after subtracting the power of very low frequency components (VLF; frequencies of <0.04 Hz). The LF/HF ratio was also calculated to evaluate the sympathovagal balance. In addition, we also evaluated the power spectral analysis of systolic BP. Systolic BP spectral powers were quantified in the LF component (0.04–0.15 Hz) and were reported in absolute units. LF of systolic BP has been associated as a reliable marker of sympathetic activity (10).
Baroreflex sensitivityBeat-to-beat values of systolic BP and RR interval were used to estimate the cardiac baroreflex sensitivity (BRS) by spectral analysis, using the alpha index for the low-frequency band (0.04 to 0.15 Hz). The coherence between the RR interval and the systolic BP signal variability was assessed by cross-spectral analysis. The alpha index was calculated only when the magnitude of the squared coherence between the RR and systolic BP signals exceeded 0.5 (range 0–1) in the LF band. After coherence calculation, the alpha index was obtained from the square root of the ratio between RR and systolic BP variability in the 2 major LF bands (10-13).
Anxiety and depression evaluationWe performed the Hamilton Scale to assess anxiety (14-15) and the Beck questionnaire to assess depression (16).
Statistical analysisThe SPSS software (SPSS 10.0, Chicago, IL) was used for statistical calculations. Data were expressed as the means±standard deviations, medians or percentages, as indicated. The Kolmogorov-Smirnov test was used to assess the normality of distribution of each variable studied. Categorical variables (sex, race and percentage of MetS components) were compared using Fisher's test. Numerical variables were compared using the unpaired Student's t test. Logistic regression analysis (forward model) was used to identify the metabolic, pro-inflammatory and prothrombotic markers that were independently associated with the MetS+BP. The model was controlled for age and BMI. ANOVA was used to select variables for the logistic regression analysis. All variables with a p<0.1 in the univariate analysis were selected for the final model. P<0.05 was considered to be statistically significant.
Ethics statementThe local ethics committee (Institutional Review Board – Heart Institute) approved the protocol, which was in accordance with the ethical standards and with the Helsinki Declaration of 1975, and all participants gave written informed consent.
RESULTSFigure 1 depicts derivation of the study sample of 75 subjects from the initial sample of 135 patients with MetS. The percentage of each MetS criterion is reported in the supplemental file.
Baseline descriptive data on the MetS patients without (N = 45) and with (N = 30) high blood pressure are provided in Table 1. We observed a significant trend for increasing age in the MetS+BP group. There was no significant difference in gender, % of Caucasians and anthropometric data, including body mass index, waist circumference, hip circumference, waist-to-hip ratio, fat percentage as calculated by skinfold, fat mass, lean mass, metabolic tax and total body water estimated by bioelectrical impedance analysis (BIA) (Table 1). As expected, there were significant differences in the systolic and diastolic blood pressure measurements between groups. Pulse intervals during spectral analysis were significantly lower (heart rate faster) in the MetS+BP compared to the MetS-BP group (Table 2). The LF components for heart rate and blood pressure and the LF/HF ratio were significantly higher in the MetS+BP group than in the MetS-BP group. Baroreflex sensitivity, as evaluated by the alpha index, was impaired in the MetS+BP group compared with the MetS-BP group. No differences were detected between groups with regards to anxiety and depression (supplemental file).
Demographic, hemodynamic and anthropometric (clinical and bioimpedance) data (means±sd) in metabolic syndrome patients without blood pressure criterion (metabolic syndrome - blood pressure) or with blood pressure (metabolic syndrome + blood pressure).
| Variables | MetS-BP (n = 45) | MetS+BP (n = 30) | p-value |
|---|---|---|---|
| Age (years) | 38±11 | 45±9 | 0.061 |
| Sex (F/M) | 34/11 | 19/11 | 0.843 |
| Caucasians, n (%) | 32 (71%) | 20 (67%) | 0.789 |
| Systolic BP (mmHg) | 121±10 | 144±18 | <0.0001 |
| Diastolic BP (mmHg) | 76±8 | 90±13 | <0.0001 |
| Heart Rate (bpm) | 72±9 | 77±12 | 0.081 |
| Body mass index (kg/m2) | 32.2±4.0 | 32.5±4.0 | 0.767 |
| Waist circumference (cm) | 108±10 (men)/100±11 (women) | 111±12 (men)/101±11 (women) | 0.6 (men) 0.7 (women) |
| Waist-to-hip ratio | 0.93±0.06 | 0.95±0.08 | 0.265 |
| Fat (%) | 32±6 | 32±7 | 0.817 |
| Fat mass (BIA), % | 28±7 | 28±9 | 0.878 |
| Lean mass (BIA), % | 59±10 | 60±13 | 0.534 |
| BMR (BIA), kcal | 1,829±328 | 1,842±398 | 0.743 |
| TBW (BIA), % | 43±8 | 44±10 | 0.73 |
BMR = basal metabolic rate; TBW = Total body water.
Power spectral analysis in metabolic syndrome patients without blood pressure criterion (metabolic syndrome - blood pressure) or with blood pressure criterion (metabolic syndrome + blood pressure).
| Variable | MetS-BP | MetS+BP | p-value |
|---|---|---|---|
| PI (ms) | 887±120 | 823±126 | 0.1 |
| VAR PI (ms2) | 2653±1910 | 2578±1529 | 0.9 |
| LF (ms2) | 638±607 | 671±498 | 0.9 |
| HF (ms2) | 687±563 | 590±549 | 0.5 |
| LF (%) | 48±15 | 56±13 | 0.04 |
| HF (%) | 52±15 | 44±13 | 0.04 |
| LF/HF | 1.0±0.7 | 1.5±0.7 | 0.08 |
| SBP (mmHg) | 126±13 | 141±21 | 0.003 |
| VAR SBP (mmHg2) | 35±25 | 50±25 | 0.03 |
| LF (mmHg2) | 6.7±4.4 | 13.0±9.2 | 0.001 |
| Alpha index (ms/mmHg) | 10.4±5.2 | 7.7±3.7 | 0.04 |
PI = Pulse interval; VAR PI = Variance of PI; LF = low frequency; HF = High frequency; SBP = Systolic blood pressure; VAR SBP = Variance of SBP.
Biochemistry data (glucose, total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, uric acid and creatinine), along with insulin and the HOMA-IR index, were significantly higher in the MetS+BP group than in the MetS-BP group (Table 3).
Biochemical data from metabolic syndrome patients without blood pressure criterion (metabolic syndrome - blood pressure) or with blood pressure criterion (metabolic syndrome + blood pressure).
| Variables | MetS-BP (n = 45) | MetS+BP (n = 30) | p-value |
|---|---|---|---|
| Glucose (mg/dL) | 97±8 | 102±7 | 0.013 |
| Insulin (U/mL) | 10.5±3.9 | 21.5±20.0 | 0.007 |
| HOMA-ir | 2.5±1.0 | 5.4±5.2 | 0.006 |
| T-cholesterol (mg/dL) | 194±33 | 221±43 | 0.001 |
| HDL-cholesterol (mg/dL) | 40.3±5.9 (men)/51.5±10.6 (women) | 38.3±6.6 (men)/42.6±7.6 (women) | 0.5 (men) 0.002 (women) |
| 119±27 | 145±39 | 0.002 | |
| Triglycerides (mg/dL) | 150±36 (men)/124±54 (women) | 190±78 (men)/167±57 (women) | 0.2 (men) 0.07 (women) |
| Uric Acid (mg/dL) | 4.6±1.2 | 5.6±1.3 | 0.001 |
| Creatinine (mg/dL) | 0.8±0.2 | 1.0±0.2 | 0.008 |
HDL = High-density lipoprotein; LDL = Low-density lipoprotein; HOMA-ir = homeostasis model assessment-estimated insulin resistance index (means±sd). Triglycerides values are reported as the medians (interquartile ranges).
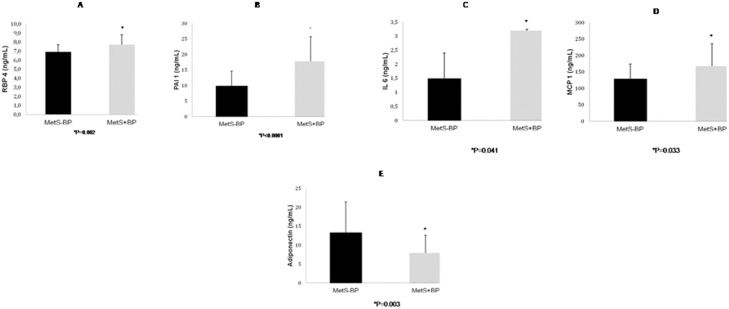
RBP4 was significantly higher in the MetS+BP group than the MetS-BP group (Figure 2A). Leptin, resistin and TNF-α levels did not differ between the MetS+BP group (49.4±41.5 pg/mL, 12.6±4.6 pg/mL and 4.8±1.7 ng/mL, respectively) and the MetS-BP group (41.4±20.0 pg/mL, 12.9±4.9 pg/mL and 4.4±1.4 ng/mL, respectively). IL-6, PAI-1 and MCP-1, a marker for inflammatory activity, were significantly higher in the MetS+BP group (Figures 2B-2D). Adiponectin was lower in the MetS+BP group compared to the MetS-BP group (Figure 2E). The differences in RBP4, PAI-1, IL-6, MCP-1 and adiponectin levels between groups remained significant after adjusting for age and sex.
Retinol-binding protein 4 levels (2A), plasminogen activator inhibitor 1 levels (2B), interleukin 6 levels (2C), monocyte chemoattractant protein 1 levels (2D) and adiponectin levels (2E) in metabolic syndrome - blood pressure and metabolic syndrome + blood pressure subjects. The results are presents as the means±SD.
Logistic regression analysis indicated that HDL, RBP-4 and PAI-1 levels were independently associated with MetS+BP. There was a strong trend for an independent association between MetS+BP and glucose levels (Table 4).
Logistic regression analysis for variables independently associated with metabolic syndrome with high blood pressure (metabolic syndrome + blood pressure)
| Variables | B | Sig. | OR | 95% C.I. for OR | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| GLIC | 0.118 | 0.057 | 1.125 | 0.996 | 1.270 |
| HDL | -0.147 | 0.008 | 0.863 | 0.775 | 0.962 |
| RBP4 | 1.507 | 0.001 | 4.511 | 1.909 | 10.663 |
| PAI1 | 0.270 | 0.002 | 1.311 | 1.103 | 1.558 |
| Constant | -20.148 | 0.011 | 0.000 | ||
Previous studies have shown that patients with MetS have multiple abnormalities that contribute to increased cardiovascular risk, including metabolic, proinflammatory, prothrombotic and autonomic variables (17-19). We hypothesized that MetS patients with elevated blood pressure may have greater abnormalities in these same variables. By stratifying patients with similar obesity levels according to the presence or absence of high blood pressure, we found that those with high blood pressure (MetS+BP) had higher surrogate markers of sympathetic activity derived from spectral analysis and greater impairment in several components of MetS, including higher glucose and triglycerides and lower HDL-cholesterol. The MetS group with high blood pressure also had higher values for other risk factors, including total cholesterol, LDL-cholesterol, uric acid and insulin, along with HOMA index and RBP4, which are associated with insulin resistance (17,20,21). In addition, several markers of inflammatory and prothrombotic activity, including IL-6, PAI-1 and MCP-1, were higher, while adiponectin levels were lower in patients with MetS and high blood pressure. Finally, we found that HDL-cholesterol, RBP4 and PAI-1 were independently associated with MetS+BP. Taken together, our results suggest a significant heterogeneity of the MetS in promoting metabolic, pro-inflammatory, prothrombotic and autonomic impairments.
MetS was defined based on the observation that a number of interrelated characteristics and diseases tended to cluster in the same individual and contributed to increased cardiovascular risk. However, MetS is not a well-defined pathophysiological entity, and the diagnostic criteria are variable and based on expert opinions (22). The literature has debated the existence of a major trigger for MetS and its components or whether the whole is really greater than the sum of the parts. Obesity emerges as an attractive candidate to explain all manifestations of MetS, but the best way to measure this parameter in the MetS scenario is also debatable. Some studies identified an association between abdominal obesity (waist circumference) and components of MetS (23,24), while others showed an association with body mass index or waist-to-hip ratio (25-28). Regardless of the obesity parameter, the importance of obesity in inducing high blood pressure and metabolic and inflammatory dysregulation is clear.
Elevated sympathetic nervous system activity seems to be crucial to the development of obesity-related risk (29). In our study, patients with MetS and high blood pressure had higher surrogate markers of sympathetic activity. Long-term sympathetic activation can lead to small artery vasoconstriction and remodeling, which increases the wall-to-lumen ratio and may act synergistically with large artery damage to raise blood pressure (30). Sympathetic activity is also related to baroreflex impairment, as observed in this study. However, sympathetic activity may not be related only to hemodynamic impairment but also to metabolic and inflammatory dysregulation.
In addition to the hemodynamic effects, sympathetically mediated vasoconstriction may also antagonize insulin-mediated glucose uptake (29). This theory is supported by longitudinal studies demonstrating that elevated indices of sympathetic activation precede the development of insulin resistance (31). Jamerson et al. showed that norepinephrine infusion, but not angiotensin, impaired insulin-stimulated glucose uptake (29-31). In line with the metabolic dysregulation observed in our study, Lichtand et al. found that autonomic nervous system dysregulation was strongly associated with MetS and its components in a large cohort of participants aged 18-65 years (35). However, these authors did not stratify patients with MetS according to blood pressure status and did not explore other risk factors not included in the MetS definition, such as proinflammatory and prothrombotic markers.
In our study, we also observed that adiponectin levels were lower and that markers of inflammation were higher in MetS patients with high blood pressure than in MetS patients without high blood pressure. Sympathetic activation reportedly reduces adiponectin release, whereas central-sympathetic blockade may increase adiponectin levels (36). Plasma adiponectin is inversely related to insulin resistance (35). Thus, the increased sympathetic activity in patients with MetS and high blood pressure may decrease adiponectin levels that, in turn, can also contribute to insulin resistance. Systemic inflammation may also be linked to sympathetic activity. Previous evidence found that indirect markers of sympathetic activity (increased heart rate and reduced heart-rate variability) were associated with subclinical inflammation in healthy middle-aged and elderly subjects. These results suggest that an autonomic imbalance in favor of the sympathetic system may interact with inflammatory processes to play a more important role in the process of vascular stiffness and atherosclerosis.
A few limitations from this investigation should be addressed. First, due to the study design, our findings suggest an association rather than a cause-effect relationship between high blood pressure and metabolic, inflammatory, prothrombotic and autonomic impairments in patients with MetS. Although consistent with the cited literature, we cannot prove that sympathetic activity is the main obesity-related trigger underlying not only the increased blood pressure but also impairments in the metabolic, inflammatory and prothrombotic profiles in this subgroup of patients with MetS. Second, direct measurements of peripheral sympathetic activity using microneurography were not performed. However, spectral analysis is an acceptable non-invasive method for estimating sympathovagal activity (13). Our findings consistently suggest a decrease in vagal activity and the activation of the sympathetic system in the spectral analyses of both heart rate and blood pressure. Third, these results cannot be extrapolated to MetS patients with diabetes. Finally, the precise reasons by which a subset of patients with MetS had higher blood pressure and greater sympathetic activity despite similar indices of obesity are not clear. Recent studies reported the potential role of psychosocial stress in inducing sympathetic activity (29,37,38). Another potential candidate is obstructive sleep apnea. This clinical condition is characterized by sympathetic activation that is independent of obesity, and it is a well-established cause of high blood pressure and increased arterial stiffness (39-41). Recent evidence in consecutive patients with MetS suggests that obstructive sleep apnea is quite common and is independently associated with increased sympathetic activity (41), metabolic and inflammatory dysregulation (42) and increased markers of atherosclerosis (43). These potential confounders should be addressed in future investigations.
In conclusion, this study provides several provocative new findings that suggest the importance of characterizing patients with MetS according to the presence or absence of high blood pressure. Although we cannot prove a cause-effect relationship, our results demonstrated that patients with MetS and high blood pressure have higher surrogate markers of sympathetic activity and significant metabolic, inflammatory and prothrombotic impairments. Thus, it is reasonable to speculate that blocking sympathetic activity is an attractive strategy for treating patients with MetS. A previous study showed that a combined α- and β-adrenergic blockade significantly reduced blood pressure in obese patients compared to lean patients with essential hypertension (44). Moreover, renal denervation, which reduces sympathetic activity, lowers blood pressure and reduces insulin resistance, may improve obstructive sleep apnea (45). Future studies will clarify whether pharmacological suppression of sympathetic activity in patients with MetS will improve not only blood pressure but also the metabolic, inflammatory and prothrombotic dysfunctions observed in these patients.
ACKNOWLEDGMENTSThis work was supported by grants from FAPESP (Fundação de Amparo à Pesquisa de São Paulo) and Zerbini Foundation.
Gil JS conceived, designed and performed the experiments; analysed the data and wrote the paper. Drager LF and Lopes HF conceived and designed the experiments, analysed the data and wrote the paper. Mostarda C performed the experiments and analysed the data. Guerra-Riccio GM and Costa-Hong V performed the experiments. Irigoyen MC and Bortolotto LA analysed the data. Egan BM wrote the paper.
No potential conflict of interest was reported.