The purpose of this paper is to evaluate the effectiveness of low-dose aspirin in the prevention of preeclampsia in low-risk and high-risk women. We identified randomized clinical trials of the use of low-dose aspirin to prevent preeclampsia through the PUBMED search engine, and through the Cochran Library database. Twenty-two studies met our inclusion criteria, and were divided according to the studied population into 2 groups: trials with women at low risk for preeclampsia and trials with women at high risk. Effects were measured through the incidence of preeclampsia in women taking either placebo or aspirin, in studies where the relative risks and the 95% confidence intervals were calculated for both groups. A total of 33,598 women were studied, comprising 5 trials with 16,700 women at low-risk and 17 trials including 16,898 women at high risk. The incidence of preeclampsia was 3.75% (626/17,700), in the low-risk group, 9.01% (1,524/16,898) in the high-risk group, and 6.40% (2,150/33,598) overall. Low-dose aspirin had no statistically significantly effect on the incidence of preeclampsia in the low-risk group (RR = 0.95, 95% CI = 0.81-1.11), but had a small beneficial effect in the high-risk group (RR = 0.87, 95% CI = 0.79-0.96). Therefore, low-dose aspirin is mildly beneficial in terms of reducing the incidence of preeclampsia in women at high risk of developing preeclampsia.
Esta revisão busca reúne estudos sobre a eficácia da aspirina em baixas doses na prevenção da pré-eclâmpsia em pacientes de alto e baixo risco. Identificamos estudos clínicos randomizados controlados usando baixas doses de aspirina para prevenir a pré-eclâmpsia, publicados no MEDLINE. Vinte e dois estudos preencheram nossos critérios de inclusão. Dividimos os estudos de acordo com a população estudada em dois grupos: estudos com mulheres de baixo risco para pré-eclâmpsia e estudos com pacientes de alto risco. A principal medida de efeito foi a incidência de pré-eclâmpsia em pacientes que usaram placebo ou aspirina, na qual os riscos relativos e os intervalos de confiança de 95% foram calculados para os grupos de pacientes de baixo e de alto risco para pré-eclâmpsia. Um total de 33.598 pacientes foram estudadas, dentre as quais cinco estudos com 16.700 pacientes de baixo risco e 17 estudos incluindo 16.898 pacientes de alto risco. As incidências de pré-eclâmpsia no geral, no grupo de baixo e no de alto risco foram de 6,40% (2.150/33.598), 3,75% (626/17.700), e 9,01% (1.524/16.898), respectivamente. Baixas doses de aspirina não tiveram efeito estatístico significante na redução da incidência de pré-eclâmpsia em pacientes de baixo risco (RR=0.95, 95%CI = 0.81-1.11), porém apresentaram pequenos benefícios em mulheres de alto risco (RR=0.87, 95%CI=0.79-0.96). Esta análise leva à conclusão de que baixas doses de aspirina têm pequeno efeito na redução da incidência da pré-eclâmpsia em pacientes com alto risco de desenvolver a doença.
Preeclampsia, defined as hypertension associated with proteinuria and/or general edema,1 continues to fascinate clinicians and basic scientists mainly because of its unknown etiological mechanisms and its complex pathophysiology. In spite of recent advances in prenatal care, preeclampsia remains a major cause of maternal and perinatal morbidity and mortality throughout the world.2 In general, preeclampsia complicates 2% to 8% of pregnancies, and it is still responsible for 10% to 15% of maternal mortality.2,3
The cause of preeclampsia remains unknown, but since the 1980s, the discovery that disordered arachidonic acid metabolism may play an important role in its pathogenesis has encouraged the use of low-dose aspirin to block this mechanism, and consequently to prevent preeclampsia.4,5 Preeclampsia is associated with a relative deficiency of intravascular production of prostacyclin (a vasodilator) and excessive production of thromboxane (a vasoconstricting prostaglandin synthesized by aggregated platelets).6 The hypothesis that antiplatelet agents such as low-dose aspirin may prevent preeclampsia has been widely tested in randomized trials. However, controversial results have been reported, with a few trials exhibiting compelling results; however, many of the largest trials to date failed to confirm any real benefit of low-dose aspirin in preventing preeclampsia.7 Additionally, it is not clear whether low-dose aspirin is beneficial only for women at high risk of developing preeclampsia, rather than also for women at low risk.
Based on this controversial condition, we reviewed the English language medical literature (MEDLINE and other data at the National Library of Medicine, accessed using the PUBMED search engine, as well as the Cochrane Library, searching for prospective studies consisting of randomized, double-blinded trials that compared the use of low-dose aspirin versus placebo to prevent preeclampsia, to evaluate the benefits of this medication in the prevention of preeclampsia either in low-risk or in high-risk populations.
DATA SOURCESStudies were identified using several search strategies such as using available electronic databases and references of either published articles or chapters from textbooks. The electronic databases used were MEDLINE and other data at the National Library of Medicine, accessed using the PUBMED search engine, as well as the Cochrane Library. We included only randomized, double-blinded trials (prospective observational studies) comparing the use of low-dose aspirin to placebo for the prevention of preeclampsia. Only completely published studies from January 1983 to December 2003 were included in this systematic review. Abstracts, unpublished trials, reviews, meta-analyses, retrospective studies, case-control studies, and trials that did not match our inclusion criteria were excluded. Each trial was individually analyzed, and included in our study if it matched our inclusion criteria.
STUDY SELECTIONTwo reviewers (R.R. and R.F.) assessed each study independently. Inclusion criteria for this investigation were randomized clinical trials that evaluated the effect of low-dose aspirin on prevention of preeclampsia in comparison with placebo.
We assessed the validity of each included trial according to the criteria outlined in the Cochrane handbook.8 Each trial received a grade for concealment of allocation, and studies with unsatisfactory quality were excluded. In all studies, data were extracted independently from each published manuscript by 1 of 2 of us (R.R. and R.F.), and data were only included and tabulated if the 2 reviewers had independently achieved the same result. When present, discrepancies were resolved by discussion, and the authors extracted the data together. However, if agreement between the authors was not possible, the study was then excluded.
In order to tabulate and integrate the data source, we had previously established that the studied populations would be divided into 2 groups: a) women at low risk of developing preeclampsia, and b) women at high risk for preeclampsia. Women at high risk of developing preeclampsia were identified as those who had essential chronic arterial hypertension prior to the pregnancy, insulin-treated diabetes, or an antecedent of severe preeclampsia (including eclampsia, HELLP syndrome, blood pressure 3 160/110 mm Hg, imminence of eclampsia, respiratory distress, or renal failure). In other studies, women at high risk for preeclampsia were identified by positive tests such as those for Doppler ultrasonography, the rollover test, or the angiotensin II sensitivity test. Women at low risk for preeclampsia were considered to be those without any risk factor or any of the positive predictive tests described above. Therefore, we first tried to select and classify each trial according to the population of interest. If the trial had mixed both populations, we decided to exclude the trial from our analysis.
Clinical heterogeneity was evaluated by the gestational age in which women were included in the study, the duration of medication and dosage of aspirin used in each trial, and the definition of high risk for preeclampsia adopted in each study (i.e. the inclusion criteria that defined being at high risk of developing preeclampsia).
The main outcome of interest to our analysis was the incidence of preeclampsia in women who had received low-dose aspirin in comparison to those who had taken the placebo, dividing these women into the 2 groups previously described (at low risk and at high risk of developing preeclampsia). As the incidence of preeclampsia was the main outcome of interest, we only included prospective longitudinal studies, excluding, as previously described, case-control ones. Although preeclampsia is defined by the onset of arterial hypertension (≥ 140/90 mm Hg) after 20 gestational weeks associated with proteinuria and/or general edema,9,10 to facilitate analysis we considered only those cases with increasing blood pressure after 20 weeks of gestation associated with proteinuria > 300 mg per 24 hours.
Tabulation of the data sources was performed using Excel® spreadsheet software (Microsoft®, USA). Two independent tables were compiled: one including low-risk, one including high-risk women. Effect was estimated by calculating the relative risk and the 95% confidence intervals; a Mantel-Haenszel fixed-effects model was used. Statistical heterogeneity was assessed using the chi-square test, and the absence of experimental intervention effect was also tested (RR = 1).
RESULTSA total of 22 randomized trials matched our inclusion criteria. Once integrated into our analysis, a total of 33,598 prospectively studied women were included. Five of these studies focused on 16,700 women at low risk of developing preeclampsia, while 17 trials followed 16,898 women at high risk (Tables 1 and 2).
Characteristics of randomized trials including women at low risk for preeclampsia
| # | Study | Location of the study | GA at inclusion | Dose of aspirin |
|---|---|---|---|---|
| 1 | Hauth et al, 199311 | USA | 20-22 w | 60 mg/day |
| 2 | Sibai et al, 199312 | USA | 13-26 w | 60 mg/day |
| 3 | Rotchell et al, 199813 | UK | 12-32 w | 75 mg/day |
| 4 | Jamaica et al, 199814 | Jamaica | 12-32 w | 100 mg/day |
| 5 | Mére-Enfant (1), 200315 | France and Belgium | 14-20 w | 100 mg/day |
#: trial; w: weeks; GA: gestation age
Characteristics of randomized trials including women at high risk for preeclampsia
| # | Study | Location of the study | GA at inclusion | Dose of aspirin | Inclusion criteria of high risk |
|---|---|---|---|---|---|
| 6 | Beaufils et al, 198516 | France | 16 w | 100 mg/day | previous history |
| 7 | Wallenburg et al, 198631 | Netherlans | 28 w | 60 mg/day | angiotensin II sensitive |
| 8 | Benigni et al, 198917 | Italy | 12 w | 60 mg/day | previous history |
| 9 | Schiff et al, 198930 | Israel | 2 8w | 100 mg/day | rollover test |
| 10 | McParland et al, 199024 | UK | 24 w | 75 mg/day | Doppler (PI > 95th centile) |
| 11 | Porreco et al, 199318 | USA | 33 w | 81 mg/day | multifetal gestations |
| 12 | Wiinikka et al, 199319 | Finland | 12-18 w | 50 mg/day | pre-existing hypertension or previous severe preeclampsia |
| 13 | CLASP, 199420 | USA | 12-32 w | 60 mg/day | clinical condition |
| 14 | Wenstrom et al, 199521 | USA | 22 w | 60 mg/day | pregestational diabetes, pre-existing hypertension, multifetal gestations, renal disease |
| 15 | ECPPA, 199622 | Brazil | 12-32 w | 60 mg/day | pregestational diabetes, preexisting hypertension, multifetal gestations, previous severe preeclampsia, renal disease |
| 16 | Morris et al, 199625 | Australia | 18 w | 100 mg/day | Doppler (S/D > 3.3 or S/D > 3 and notch) |
| 17 | Bower et al, 199626 | UK | 18-22 w | 60 mg/day | Doppler (PI > 95th centile) |
| 18 | Zimmermann et al, 199727 | Italy | 22-24 w | 50 mg/day | Doppler (bilateral notch) |
| 19 | Caritis et al, 199823 | USA | 13-26 w | 60 mg/day | pregestational diabetes, pre-existing hypertension, multifetal gestations, previous severe preeclampsia |
| 20 | Harrington et al, 200028 | UK | 17-23 w | 100 mg/day | Doppler (RI > 50% and bilateral notch or RI > 90% and unilateral notch or RI > 95%) |
| 21 | Mére-Enfant (2), 200329 | France and Belgium | 14 w | 100 mg/day | Doppler (PI > 95th centile) |
| 22 | Yu et al, 200332 | Different countries | 23 w | 150 mg/day | Doppler (PI > 95th centile) |
#: trial; w: weeks; GA: gestational age.
Good agreement between reviewers for selection and assessment of validity was observed, and discrepancies were easily resolved. Only trials with satisfactory concealment of allocation were included.
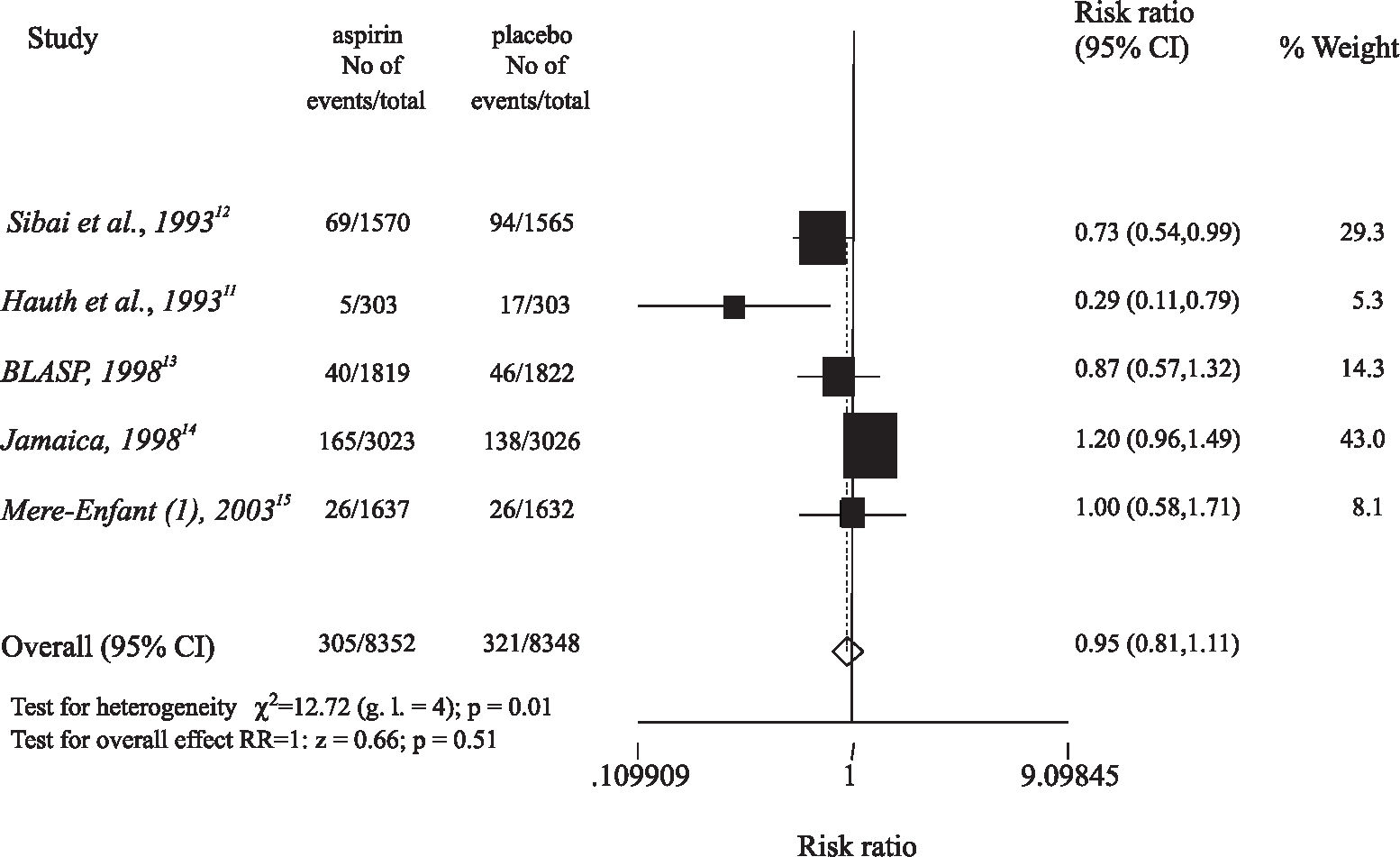
Among the 5 trials that evaluated women at low risk for preeclampsia (Table 1), 2 studies used 60 mg/day of aspirin,11,12 1 used 75 mg/day,13 and 2 used 100 mg/day.14,15 Four trials started giving aspirin to women in the first trimester,12–15 and 1 study included women in the second trimester.11 In the first 2 trials,11,12 a significant reduction in the incidence of preeclampsia was observed among those women who received aspirin (RR = 0.73 and 95% CI = 0.54-0.99, RR = 0.29 and 95% CI = 0.11-0.79, respectively), but the 3 latter studies did not demonstrate any benefit of low-dose aspirin (Figure 1).
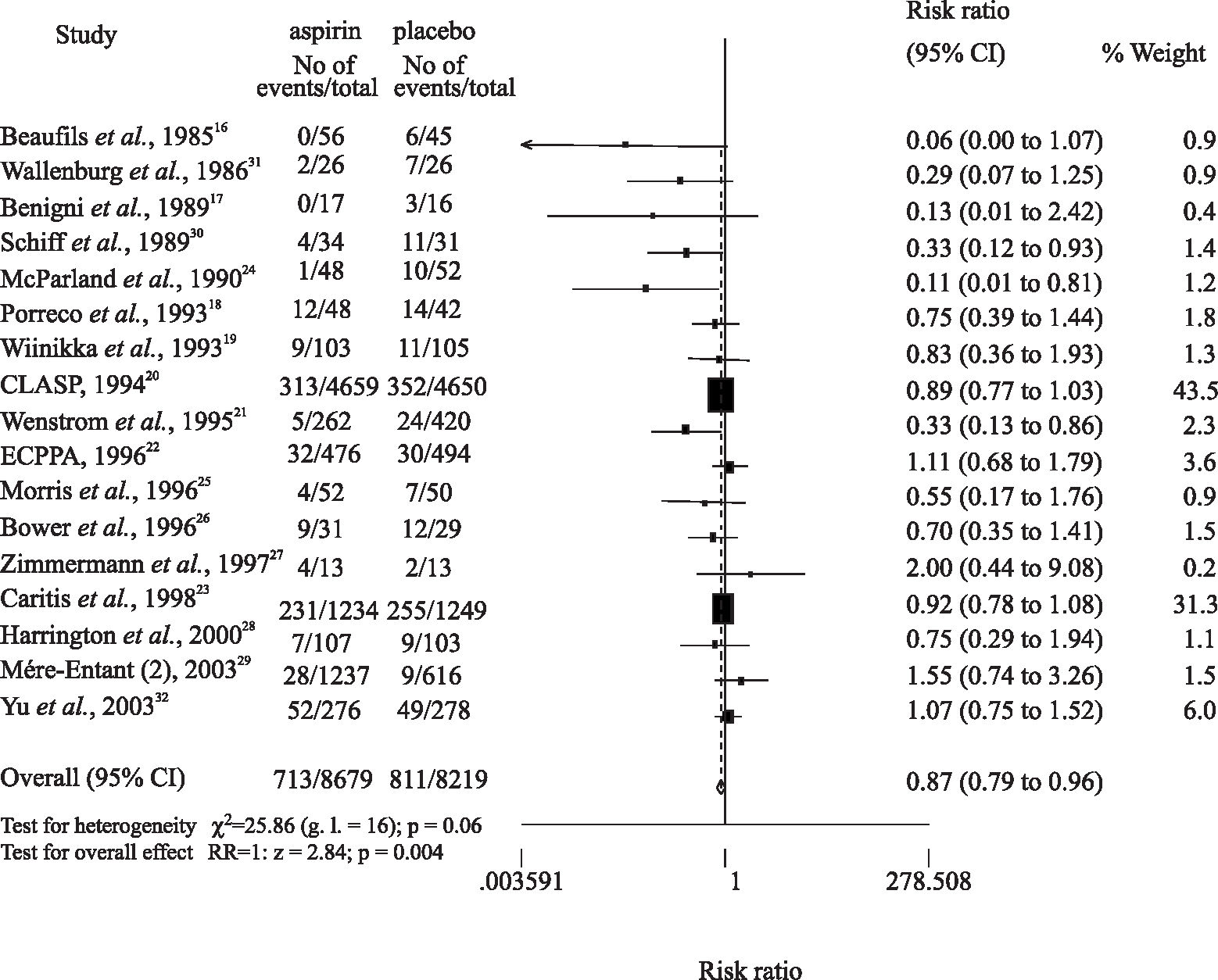
Among the 17 trials that evaluated women at high risk for preeclampsia (Table 2), the various main inclusion criteria were as follows: previous maternal history (2 studies)16,17; pregestational maternal disease, previous severe preeclampsia, or multiple gestation (6 studies)18–23; an abnormal Doppler examination (7 studies)24–29,32; positive rollover test (1 study)30; and a positive angiotensin II sensitivity test (1 study).31 Regarding aspirin dosages given to high-risk women, the following variations occurred: 50 to 60 mg/day (9 studies)17,19–23,26,27; 75 mg/day (1 study)24; 81 mg/day (1 study)18; 100 mg/day (5 studies)16,25,28–30; and 150 mg/day (1 study).32
In 11 trials, women started taking aspirin in the second trimester,16,18,21,24–28,30–32 while in 6 studies women started taking aspirin in the first trimester.17,19–23,29 In 9 studies, primarily those in the former group, a preventive role of low-dose aspirin was observed, with a relative risk varying from 0.06 to 0.7516–18,21,24–26,28,30,31; others showed no considerable benefits of the low-dose aspirin in preventing preeclampsia19,20,22,27,23,29,32 (Figure 2).
The overall incidence of preeclampsia was 6.40% (2,150/33,598). The incidences of preeclampsia in the low-risk and in the high-risk populations were 3.75% (626/16,700) and 9.01% (1,524/16,898), respectively.
In women at low risk of developing preeclampsia, 8,352 women received prophylactic low-dose aspirin while 8,348 women took a placebo. Preeclampsia was observed in 305 (3.65%) women who took low-dose aspirin, and in 321 (3.85%) women who took a placebo (RR = 0.95, 95% CI = 0.81-1.11) (Figure 1).
In women at high risk of developing preeclampsia, 8,679 women took low-dose aspirin while 8,219 took a placebo. Preeclampsia occurred in 713 (8.22%) women who received low-dose aspirin, and in 811 (9.87%) women who took a placebo (RR = 0.87, 95% CI = 0.79-0.96) (Figure 2).
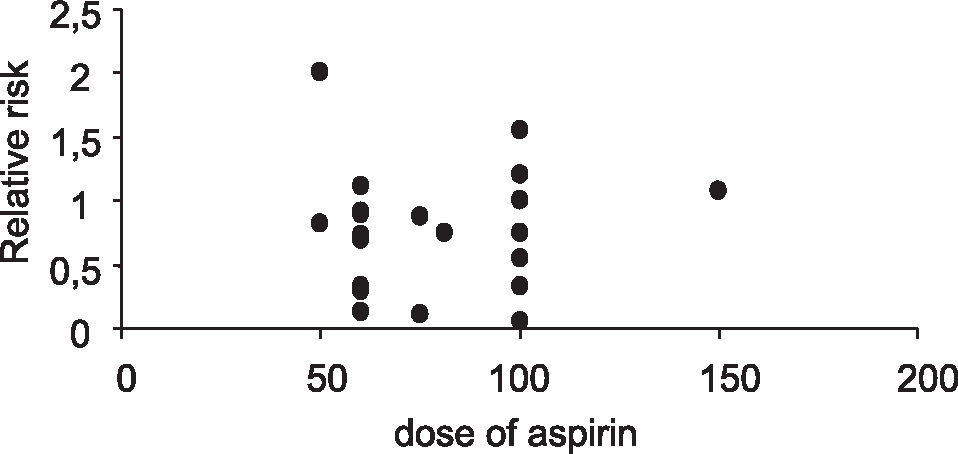
Although different doses of aspirin were used in each trial, there was no correlation between dose of aspirin and prevention of preeclampsia (correlation coefficient r = 0.064, Figure 3).
DISCUSSIONOur systematic review and meta-analysis shows that low-dose aspirin has a small effect in the prevention of preeclampsia in women considered to be at high risk for the disease (RR = 0.87). The analysis confirms the tendency of recent randomized trials that have been demonstrating the small effect of low-dose aspirin in the prevention of preeclampsia.
The validity of this analysis depends on the methodological strength of our review. We used a prospective protocol and attempted to find all the evidence. Only randomized controlled studies that were completely and clearly described were included in our meta-analysis, because they are the most reliable method of assessing efficacy of therapeutic interventions in the incidence of a special disease. To exclude interpreted data, previous reviews and meta-analyses were not included. Since an extremely important issue related to meta-analysis is the quality of studies, we considered only studies with high quality, which may have reduced the number of studies analyzed in our review.33
Another important issue in a meta-analysis is the degree of heterogeneity of design of the included studies. Excessive heterogeneity reduces the validity of the approach. On the other hand, although absolute design homogeneity among studies would be ideal and desirable, this is an almost impossible goal to achieve. However, the problem can be avoided by understanding and analyzing the sources of heterogeneity.33
To achieve this, we first, established that our principal measure of effect would be the incidence of preeclampsia, defined as a blood pressure increase after 20 weeks of gestation associated with proteinuria ≥ 300 mg per 24 hours. Gestational hypertension (increasing blood pressure without proteinuria) was not considered to be an indication of preeclampsia. Additionally, we did not evaluate other outcomes, such as prematurity, or fetal growth restriction, as we believe that these may be consequences of preeclampsia.
Differences in aspirin dosage and duration of treatment were also observed among studies. Earlier studies tended to use lower doses of aspirin (50-75 mg/day), while more recent trials used 100 mg/day aspirin. Wallenburg34 pointed out that the difference between early and recent trials regarding the aspirin dosage have influenced the results. In his opinion, daily doses of 60 to 80 mg of aspirin effectively inhibit platelet thromboxane synthesis, but these may not be sufficient to affect placental thromboxane. Nevertheless, in the present review, the more recent trials have not disclosed any benefit in the prevention of preeclampsia with the higher dose of aspirin (100-150 mg/day), a fact confirmed in our analysis by demonstrating that differences in aspirin dosage did not correlate with the relative risk for preeclampsia.
Although little attention has been paid in published trials to the timing of aspirin administration (duration of treatment), some studies suggest greater efficacy when aspirin treatment begins before 17 or 20 weeks,7,20,35,36 while others find no benefits with earlier treatment.13,15,23,29,37 In this study, we observed no obvious correlation between timing of aspirin administration and prevention of preeclampsia, although this point could not be definitely evaluated because the timing of aspirin administration varied between, and even within a number of trials. The duration of aspirin administration should be carefully evaluated in all future clinical trials.
The last and most important cause of clinical heterogeneity was the inclusion criteria of high, or low risk of developing preeclampsia (Tables 1 and 2). As Table 2 demonstrates, different inclusion criteria were used in each trial to define women at high risk for preeclampsia. Previous severe preeclampsia, preexisting hypertension, pregestational diabetes, renal disease, and multifetal gestations are well known risk factors for preeclampsia.37–39 However, in many studies, all these conditions, which may have different pathophysiology, are mixed together,. Therefore, it should be of interest to randomize women according to each different risk factor in further trials. Additionally, the positive rollover test and the positive angiotensin II sensitivity test were the inclusion criteria in 2 trials.30,31 In 7 trials, abnormal uterine Doppler indexes were used to recruit participants.24–29,32 These tests are considered to be methods of predicting preeclampsia, although their reported accuracy is variable.40–42 In addition, although nulliparity is theoretically considered to be a risk factor for preeclampsia,39 in all but 1 study, 15 nulliparity was not considered to be a high-risk inclusion criterion. In the present review, among the 5 trials included in the low risk-group, 4 studied healthy nulliparous women,11,12,14,15 and 1 included 44% nulliparous women.13
Considerations of cost and effects of preeclampsia prevention are justified by its high prevalence and by the associated high maternal and perinatal morbidity/mortality rates.34 Primary prevention, consisting of averting the occurrence of the disease, requires an understanding of its etiology. As the fundamental problem of misalliance between trophoblast and maternal tissue is not well understood, true etiological intervention has not been possible in preeclampsia. On the other hand, secondary prevention, consisting of at least slowing down the disease process before it becomes clinically apparent, is based on pathophysiological concepts. Low-dose aspirin has been used as a strategy for secondary prevention of preeclampsia.34 However, as observed in recent studies and in the present analysis, low-dose aspirin exhibits nothing but a small effect in the prevention of preeclampsia, and this benefice is restrcited to the high-risk group, showing that we still need to study and better understand the pathophysiology of the preeclampsia.
Causes of conflicting results may not be explained by the timing of aspirin administration and its dosage, but more probably by differences in the inclusion criteria. Additionally, methods of prediction of preeclampsia seem to have questionable results. Recently, many severe cases of preeclampsia have been associated with thrombophilia and antiphospholipid syndromes,44–52 situations in which low-dose of aspirin seems to have beneficial effects. As these diseases were not routinely screened for, many trials included these women, without realizing the fact, which may explain some of the good results with low-dose aspirin in the prevention of preeclampsia in earlier studies.
Therefore, in further randomized controlled trials evaluating the use of low-dose aspirin, participants should be divided into groups according to parity, risk for preeclampsia, and the presence of any prothrombotic factor or disease. Because secondary prevention is based on pathophysiology, we believe our analysis shows that pathophysiological process involved in preeclampsia still require further study.