Hypoplastic left heart syndrome (HLHS) always results in death if not surgically treated. Approximately half of the children diagnosed with HLHS are not referred for surgery and die within days or weeks (1–3). The surgical treatment of HLHS includes three highly complex, costly surgeries that have a high mortality rate (4). The mortality rate during the first stage of the surgery ranges from 25 to 80%. The Norwood procedure, the first stage of surgical correction, uses two conventional techniques to construct a systemic pulmonary shunt. The Norwood procedure with a Blalock-Taussig shunt provides systemic pulmonary blood flow but it physiologically mimics systemic aortic valve insufficiency during diastole. The Sano modification of the Norwood procedure consists of placing a tube between the systemic ventricle and the pulmonary arteries. This technique prevents diastolic systemic pulmonary blood reflow but causes ventricular reflux, leading to volume overload. This overload (associated with the ventriculotomy) may lead to ventricle dysfunction and arrhythmia. Both techniques necessarily use an artificial tube, which increases the risk for thrombosis or stenosis (5–9). Moreover, creating the neo-aorta is a laborious procedure that involves the prolonged use of extracorporeal circulation (ECC).
A hybrid approach simplifies the surgery by maintaining the patent ductus arteriosus (DA) with a stent implantation (10,11) or through the prolonged use of prostaglandin E1 (12), associated with the banding of the pulmonary branches. However, the results of this approach have proven unsatisfactory (13,14).
CASE DESCRIPTIONThis study was approved by the Research Ethics Committee at the Jundiai Medical School in Jundiaí, São Paulo, Brazil. The risks and mortality related to treated and untreated HLHS were fully explained to the parent of the patient. All questions were answered and an informed consent form was signed.
A male newborn was delivered with a pre-natal diagnosis of HLHS in the Paulo Sacramento Hospital, Jundiaí, São Paulo, Brazil. An echocardiographic examination confirmed the following pathology: HLHS with atresia of the mitral and aortic valves, a hypoplastic left ventricle, a large hypertrophic right ventricle, a large nonrestrictive atrial communication, a large main pulmonary artery with a large DA, and a small caliber (3.0 mm) hypoplastic ascending aorta.
The patient presented with discrete cyanosis with 95% arterial oxygen saturation (without supplementary oxygen) and mild respiratory distress. Intermittent continuous positive air pressure (oxygen at 21%) was used in the first three days of life. Prostaglandin was used continuously to maintain the DA open. Surgery was performed on the seventh day of life when the patient weighed 3.2 kg.
The usual preparations were made for an intracardiac operation with ECC. Anesthesia was induced with ketamine 1 mg/kg, fentanyl 5 υg/kg, and pancuronium 0.1 mg/kg. The patient received orotracheal intubation with a 3 mm tube. Sevoflurane 2% and air was used to maintain anesthesia.
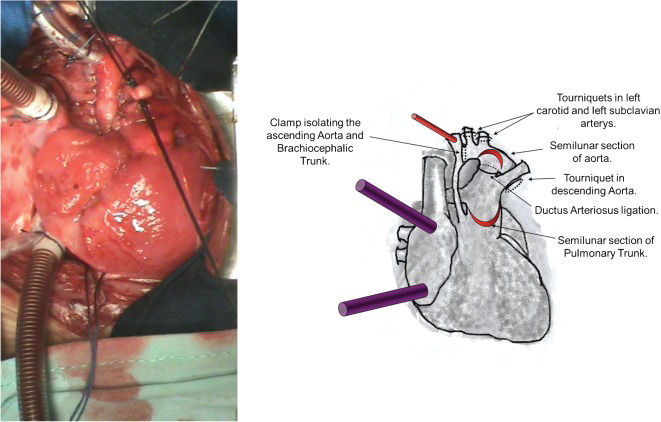
A conventional median sternotomy was performed, along with the dissection and cannulation of the brachiocephalic trunk. The 8 French cannula was oriented toward the atretic aortic valve. Both venae cavae were cannulated. Tourniquets were placed on the left carotid artery, the left subclavian artery and the descending aorta (after the insertion of the DA).
ECC was initiated with 80 ml/kg/min flow, and temperature was maintained at 35°C. The patient was infused with mannitol 1 ml/kg, dexamethasone 0.1 mg/kg and sodium bicarbonate 8.4% 1 mEq/kg.
The DA was ligated (Figure 1), and the ECC flow was temporarily reduced to 20 ml/kg/min. A tourniquet was placed at the aortic arch, isolating the ascending aorta and the brachiocephalic trunk from the systemic circulation and thus preserving only the cerebral, right superior limb and coronary perfusion. The left carotid artery, left subclavian artery and descending aorta tourniquets were closed.
Photograph and diagrammatic schema of the cannulation of the brachiocephalic trunk and double venae cavae for extracorporeal circulation (ECC). A tourniquet was placed in the left carotid, the left subclavian artery and the descending aorta. ECC was initiated, and the DA was ligated. A clamp was placed at the aortic arch, isolating the ascending aorta and the brachiocephalic trunk from the systemic circulation. The tourniquets were then closed. A flap was created in the semilunar section in the initial portion of the anterior pulmonary trunk 3 cm above the pulmonary valve. A second semilunar incision was made in the anterosuperior side of the arch of the aorta near the left carotid and left subclavian arteries, creating the second flap.
The technical modification proposed by Rocha-e-Silva (15) was a semilunar section in the initial portion of the anterior pulmonary trunk, 3 cm above the pulmonary valve. This modification created a flap of autologous tissue (Figure 1). A second semilunar incision was made in the anterosuperior side of the aortic arch near the left carotid and the left subclavian artery, creating a second flap of autologous tissue (Figure 1). After opening the aortic arch, a cannula was placed through the tourniquet of the descending aorta to allow for systemic perfusion; the ECC flow was then increased to 60 ml/kg/min.
We introduced a 5 mm catheter through the opening of the pulmonary trunk toward the pulmonary branches. Around this catheter, we placed the pulmonary trunk cerclage using cardiac tape sutured with 5-0 Prolene. This procedure resulted in a systemic pulmonary shunt with autologous tissue (Figure 2).
The free edges of the semilunar flaps of the aorta and pulmonary trunk were rotated and sutured edge-to-edge, originating the autologous posterior neo-aortic wall (Figure 3). A second line of stiches was placed to dorsally exclude the DA tissue.
Because the descending aortic cannula occupied too much space, it was removed to suture the anterior neo-aortic wall patch. During this interruption, systemic perfusion was interrupted, and the ECC flow was temporarily reduced to 20 ml/kg/min.
A valved bovine pericardium patch was implanted from the anterior opening of the pulmonary trunk to the aortic arch, extending to the beginning of the descending aorta. This procedure resulted in the anterior wall of the neo-aorta (Figure 4). The opening of the valve was placed at the lower edge of the aortic arch. This valve prevents diastolic systemic reflux to the pulmonary arteries and improves coronary perfusion pressure through the ascending aorta (retrograde flow). The neo-aorta did not require mobilization of either the ascending aorta or the pulmonary trunk.
A small right atriotomy was performed for an atrial septum resection. After the air was removed, the tourniquets were released for the subsequent finalization of ECC, which lasted for 95 min.
The heart maintained its systolic function throughout the procedure and fully recovered arterial pressure by the end of the ECC. The blood volume remaining in the circulatory system was uneventfully re-transfused, and the cannulas were removed. A small dose infusion of 5 mcg/kg/min dopamine was started. Because the systemic pulmonary shunt was valved, the ventilation FiO2 was maintained at 60%. The patient was infused with protamine, and any bleeding was minimal.
After 135 minutes (commencing with the interruption of ECC) and with only 5 mcg/kg/min of dopamine, the blood samples showed that blood gas, pH base excess and hemoglobin/hematocrit levels were adequate (Table 1).
Blood gas levels, electrolyte concentrations, and hemoglobin and hematocrit levels throughout the modified Norwood procedure.
| INITIAL | ECC initial | ECC final | POST-ECC15 min | POST-ECC75 min | POST-ECC135 min | |
|---|---|---|---|---|---|---|
| pH | 7.038 | 7.570 | 7.682 | 7.172 | 7.307 | 7.326 |
| PCO2 (mmHg) | 105.1 | 26.5 | 13.3 | 56.3 | 57.9 | 67.5 |
| cHCO3 (mmol/l) | 27.6 | 23.7 | 15.4 | 20.2 | 28.3 | 34.4 |
| BE∗ (mmol/l) | -5.8 | 2.4 | -2.6 | -8.5 | 1.1 | 5.9 |
| PO2 (mmHg) | 136.0 | 219.8 | 194.8 | 77.8 | 51.3 | 85.0 |
| SO2 (%) | 96.7 | 97.6 | 98.0 | 88.4 | 78.7 | 92.9 |
| SO2 (C) (%) | 96.9 | 99.9 | 99.9 | 90.2 | 82.2 | 95.7 |
| ctCO2 (P) (mmol/L) | 30.9 | 24.5 | 15.8 | 21.9 | 30.1 | 36.5 |
| Na+ (mmol/L) | 131.6 | 139.8 | 144.2 | 143.9 | 147.4 | 156.6 |
| K+ (mmol/L) | 5.15 | 5.47 | 5.62 | 4.06 | 3.88 | 4.16 |
| Cl- (mmol/L) | 100.6 | 104.7 | 103.2 | 105.3 | 109.1 | 102.6 |
| iCA++ (mmol/L) | 1.447 | 0.645 | 0.956 | 1.018 | 0.953 | 0.989 |
| Hb (g/dl) | 12.9 | 10.1 | 9.3 | 11.0 | 10.3 | 11.3 |
| Hct (%) | 40.0 | 30.6 | 27.7 | 33.8 | 30.4 | 34.2 |
ECC: Extra corporeal circulation. BE: Base excess.
When wound closure was begun, an electrocardiographic change with hypotension was noticed. Adrenalin 0.1 mcg/kg/min was initiated. Approximately 15 min after the wound closure was completed, there was a rapid and progressive deterioration of the heart with cardiac arrest, which proved non-responsive to all intervention.
DISCUSSIONThis new technique for correcting HLHS uses a smaller number of sutures and simplifies the procedure by excluding the implantation of a heterologous systemic pulmonary shunt, decreasing surgical time and potentially reducing perioperative complications.
The described technique does not require any period of coronary ischemia or surgical manipulation of the ventricle. The combination of these factors should promote a better quality of postoperative ventricular function. The manner in which the ventricle recovered its pumping function after ECC reinforces this concept.
Maintaining the anatomy of the ascending aorta simplifies the procedure while avoiding any distortion in the coronary arteries.
The autologous systemic pulmonary shunt is simple to manufacture and should not cause any thrombosis problems. In addition, the maintenance of the pulmonary trunk in situ (with the cerclage) should promote good development of the pulmonary tree.
The use of a valved pericardium to create the neo-aorta should improve systemic perfusion (including coronary circulation) and prevent diastolic systemic pulmonary reflux. The full technique, including the changes suggested in this discussion, is described elsewhere (15).
As stated above, 135 minutes after the interruption of ECC, and with only 5 mcg/kg/min of dopamine infusion, the arterial pressure was normal, and blood gases were satisfactory. At this point, the sudden deterioration of cardiac function led to the death of the patient. A massive coronary thrombosis is the most likely hypothesis for this sudden deterioration. The placement of the brachiocephalic trunk cannula toward the atretic aortic valve may have induced a preferential flow to the coronary arteries. Because its caliber was nearly commensurate with that of the brachiocephalic trunk, the right brachial pressure may have underestimated the pressure in the ascending aorta, which may have led to a coronary endothelial washout. A solution for this problem is the dissection and placement of a 3.5 mm Gore-Tex vascular graft implantation in the brachiocephalic trunk for ECC arterial access or the use of a 6 French cannula oriented cranially.
Placing and removing the descending aortic cannula takes time and does not ensure a continuous perfusion of the descending aorta. The femoral artery should be dissected and cannulated to provide secondary arterial access to maintain continuous systemic perfusion, even during aortic clamping.
The use of this technique can bring promising results.
AUTHOR CONTRIBUTIONSRocha-e-Silva R, De Mola R, Santos ES and Martins DM performed the surgery. Pesciotto VR was the chief cardiologist. Hatori DM and Greco JP administered the anesthesia.
No potential conflict of interest was reported.