The impact of Multivisceral Liver Resection (MLR) on the outcome of patients with Colorectal Liver Metastasis (CRLM) is unclear. The present systematic review aimed to compare patients with CRLM who underwent MLR versus standard hepatectomy regarding short- and long-term outcomes. MLR is a feasible procedure but has a higher risk of major complications. MLR did not negatively affect long-term survival, suggesting that an extended resection is an option for potentially curative treatment for selected patients with CRLM.
The incidence of colorectal cancer has been increasing in recent decades, reaching more than 1,930,000 newly diagnosed cases in 2020.12 Currently, colorectal cancer is the second most frequent cause of cancer-associated mortality worldwide with approximately 935,000 deaths annually.2 The liver is the most common site of metastatic spread (up to 80% of patients).3 Approximately 50% of patients with colorectal cancer will develop liver metastases during follow-up, and 15% to 25% of these patients will be diagnosed with their primary tumors.34 The incidence of Colorectal Liver Metastasis (CRLM) is approximately 4.3% in 1-year, 8.7% in 2-years, 12.7% in 3-years, and 16.5% in 5-years.5 Importantly, the metastatic disease has a significant prognostic impact, accounting for two-thirds of deaths in patients with colorectal cancer.6
The cornerstone of CRLM treatment is the combination of systemic chemotherapy and complete resection of liver lesions with clear surgical margins (R0 resection), resulting in a 5-year Overall Survival (OS) of 40%‒60%.7–9 However, the lesions of only 20%‒25% of patients with CRLM are considered resectable at initial presentation.10
Surgical margins are a major issue in the surgical treatment of CLRM. Several studies have shown that microscopic-free surgical margins offer long-term benefits compared to R1 resections.11–16 Therefore, for patients with locally advanced CRLM involving adjacent organs or structures, hepatectomy combined with resection of the involved adjacent organs/structures is necessary to achieve free surgical margins.17
However, the impact of Multivisceral Liver Resection (MLR) on patients with CLRM who underwent surgical treatment is unclear. Some studies have shown a negative impact of multi-visceral resections on perioperative morbidity and significantly worse long-term outcomes,1819 while other studies have failed to detect any difference comparing MLR and standard hepatectomy.1720 Despite the increased performance, the available evidence that supports MLR in patients with CRLM is from retrospective cohorts1920 and comparative studies with underpowered small sample sizes.17–20 To date, no systematic review or meta-analysis has been published on this topic, indicating that quality data supporting the indications, feasibility, and oncological outcomes of MLR are lacking.
The present study aimed to compare the short- and long-term outcomes of patients with CRLM who underwent MLR versus standard hepatectomy with curative intent.
MethodsThe present study was approved by the Institutional Ethics Committee and conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.21 This research protocol was registered in the International Prospective Register of Systematic Reviews (http://www.crd.york.ac.uk/PROSPERO) under number CRD42021244265.
Database searchA systematic review was performed in PubMed, Embase, Cochrane Library Central, Scientific Library Electronic Online/Latin American and Caribbean Health Sciences Literature (SciELO/LILACS), and grey literature by two independent authors. Databases were searched for Randomized Controlled Trials (RCTs) and comparative observational studies that evaluated the perioperative and long-term outcomes of patients who underwent MLR or standard hepatectomy for CRLM with curative intent. The search was limited to human subjects and included prospective and retrospective studies regardless of language or date of publication. Retrieved references were cross-checked manually for additional studies. The last search was performed on June 09, 2022.
The search strategy in PubMed was based on the following Medical Subject Headings (MeSH) and keywords: ((((multivisceral) OR (extended) OR (diaphragm) OR (stomach) OR (gastric) OR (gastrectomy) OR (inferior vena cava) OR (kidney) OR (nephrectomy)) AND (((hepatectomy) OR (hepatectomies) OR (liver resection)) AND (((colorectal) OR (rectal) OR (colonic)) AND ((neoplasm) OR (cancer) OR (tumour) OR (carcinoma) OR (adenocarcinoma)))))). For EMBASE, Cochrane Library Central, and SciELO/LILACS, the search was performed with the same keywords in various combinations.
Study selectionThe study selection was performed by two independent reviewers. Any disagreement on the inclusion or exclusion of a given study was resolved by a consensus meeting. Initially, titles and abstracts were screened, and irrelevant (or duplicate) studies were excluded; the full text of potentially eligible articles was then analyzed. The following inclusion criteria were used: (1) RCTs and observational studies (prospective or retrospective) that compared perioperative and/or long-term outcomes of patients with CRLM who underwent MLR or standard hepatectomy; and (2) The definition of MLR was any hepatectomy with en bloc resection of at least one adjacent organ or structure, including extrahepatic vascular resections (e.g., Inferior Vena Cava [IVC] and/or hepatocaval confluence) not usually performed in a standard hepatectomy. Associated resection of the gallbladder, hepatic pedicle structures (hepatic artery, portal vein, and biliary tree), and simultaneous resection of gastrointestinal tumors and synchronous liver metastasis without direct invasion of the liver by the primary tumor was not considered MLRs.22 If the same patients were included in more than one study, the most recent or the one of higher quality was selected.
The exclusion criteria were as follows: (1) Noncomparative studies, review articles, letters, and case reports; (2) Studies with other definitions of MLRs; (3) Studies with missing values or data for outcome calculation; and (5) Studies unavailable in full text.
Data extractionFull text, tables, and figures of selected studies were assessed for data extraction. The following data were collected: (1) Name of the first author and year of publication; (2) Study type; (3) Number of patients per group; (4) Patient characteristics, including age and sex; (5) Type of liver resection and type and number of adjacent organs/structures resected; and (6) Outcomes, including operative time, estimated blood loss, blood transfusion rate, length of hospital stay, frequency of compromised margins, overall morbidity, and 30-day perioperative mortality. Perioperative morbidity was stratified according to the Clavien-Dindo classification.23
Level of evidence and quality assessmentStudy quality was assessed using Robins-I,24 and certainty assessment was performed using Grading of Recommendations Assessment, Development, and Evaluation (GRADE) recommendations.25
Statistical analysisThe meta-analysis was performed using STATA 16.1 software. Continuous variables are expressed as the mean ± SD and summarized as the Mean Difference (MD) and 95% Confidence Interval (95% CI). Categorical variables are expressed as absolute numbers and summarized as Risk Differences (RDs) and 95% CIs. Hazard Ratios (HRs) and their corresponding lower and upper 95% CI limits were extracted from the individual time-to-event outcomes of the included studies. When the HR and associated standard error or CI were not provided, the HR was calculated using different statistical methods based on the clinical and statistical data reported in the primary studies.2627
Study heterogeneity was assessed using chi-square and I2 statistics. A random-effects analysis model was applied to adjust for expected interstudy heterogeneity to provide a more conservative CI around the pooled HR.28 Because no more than ten studies were included in the meta-analysis, publication bias evaluation was not performed due to the low power of the funnel plot test to distinguish chance from real asymmetry.
Whenever possible, subgroup analysis according to the type of extrahepatic organ/structure resected was performed. The significance level was set at 5% (p < 0.05).
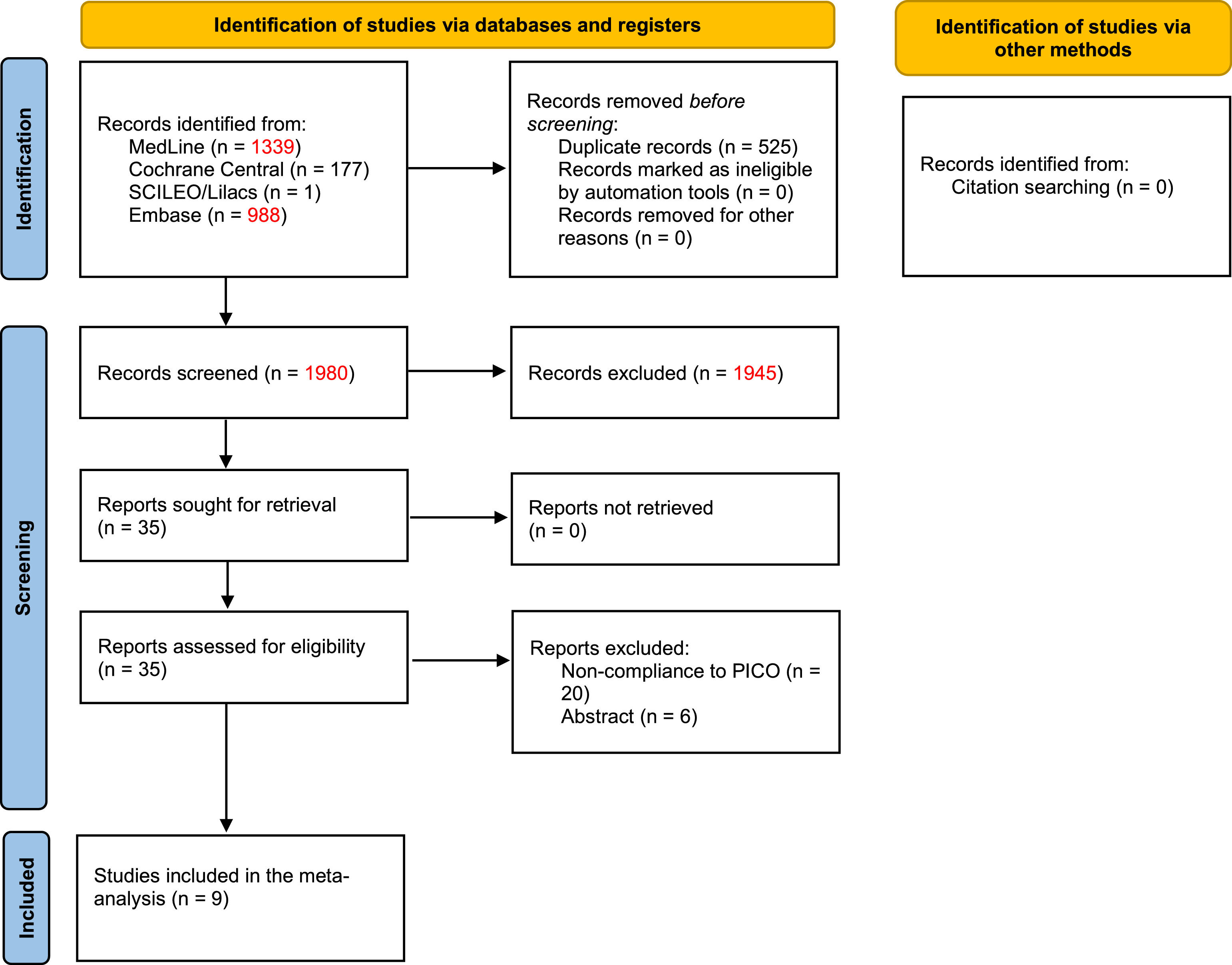
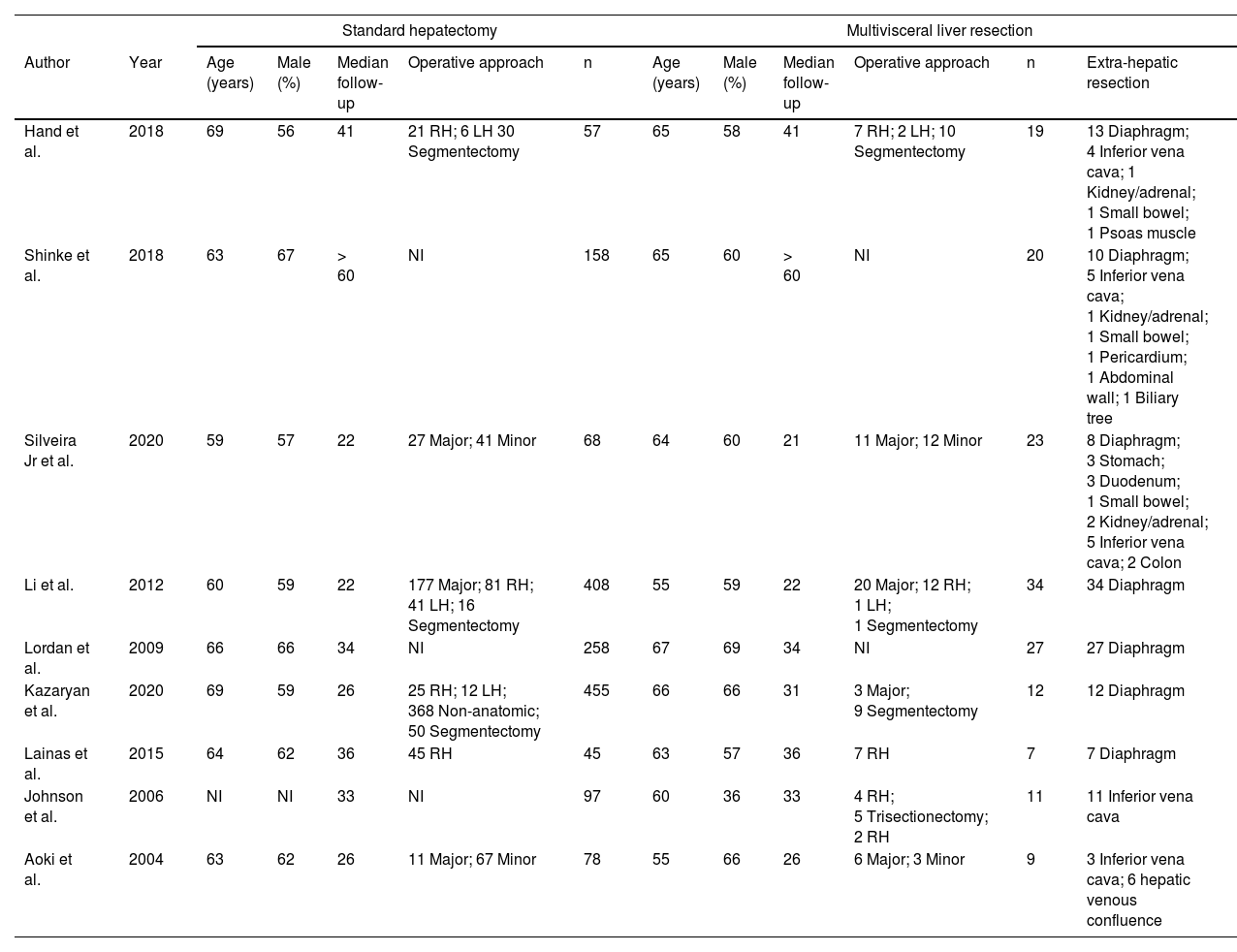
ResultsBaseline characteristicsOf the 1,980 initially screened articles, 9 comparative studies (comprising 1,786 patients) were included in the systematic review (Fig. 1).17-20,22,29-32 All of the included studies were observational, and no RCTs were found. A previous case-match study published by the present group compared the outcomes of MLR vs. standard hepatectomy; however, the CRLM subgroup was evaluated only for long-term outcomes.22 The raw data of this subgroup were retrieved and included in the quantitative analyses of perioperative outcomes. The baseline characteristics of the included studies are shown in Table 1. The assessment of certainty and risk of bias are shown in Supplementary Files 1 and 2, respectively.
Baseline characteristics of the included studies.
| Standard hepatectomy | Multivisceral liver resection | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Age (years) | Male (%) | Median follow-up | Operative approach | n | Age (years) | Male (%) | Median follow-up | Operative approach | n | Extra-hepatic resection |
| Hand et al. | 2018 | 69 | 56 | 41 | 21 RH; 6 LH 30 Segmentectomy | 57 | 65 | 58 | 41 | 7 RH; 2 LH; 10 Segmentectomy | 19 | 13 Diaphragm; 4 Inferior vena cava; 1 Kidney/adrenal; 1 Small bowel; 1 Psoas muscle |
| Shinke et al. | 2018 | 63 | 67 | > 60 | NI | 158 | 65 | 60 | > 60 | NI | 20 | 10 Diaphragm; 5 Inferior vena cava; 1 Kidney/adrenal; 1 Small bowel; 1 Pericardium; 1 Abdominal wall; 1 Biliary tree |
| Silveira Jr et al. | 2020 | 59 | 57 | 22 | 27 Major; 41 Minor | 68 | 64 | 60 | 21 | 11 Major; 12 Minor | 23 | 8 Diaphragm; 3 Stomach; 3 Duodenum; 1 Small bowel; 2 Kidney/adrenal; 5 Inferior vena cava; 2 Colon |
| Li et al. | 2012 | 60 | 59 | 22 | 177 Major; 81 RH; 41 LH; 16 Segmentectomy | 408 | 55 | 59 | 22 | 20 Major; 12 RH; 1 LH; 1 Segmentectomy | 34 | 34 Diaphragm |
| Lordan et al. | 2009 | 66 | 66 | 34 | NI | 258 | 67 | 69 | 34 | NI | 27 | 27 Diaphragm |
| Kazaryan et al. | 2020 | 69 | 59 | 26 | 25 RH; 12 LH; 368 Non-anatomic; 50 Segmentectomy | 455 | 66 | 66 | 31 | 3 Major; 9 Segmentectomy | 12 | 12 Diaphragm |
| Lainas et al. | 2015 | 64 | 62 | 36 | 45 RH | 45 | 63 | 57 | 36 | 7 RH | 7 | 7 Diaphragm |
| Johnson et al. | 2006 | NI | NI | 33 | NI | 97 | 60 | 36 | 33 | 4 RH; 5 Trisectionectomy; 2 RH | 11 | 11 Inferior vena cava |
| Aoki et al. | 2004 | 63 | 62 | 26 | 11 Major; 67 Minor | 78 | 55 | 66 | 26 | 6 Major; 3 Minor | 9 | 3 Inferior vena cava; 6 hepatic venous confluence |
NI, Not Informed; RH, Right Hepatectomy; LH, Left Hepatectomy.
The mean age was 64 years with a male predominance (61%), and the mean postoperative follow-up was 31 months. Subgroup analysis was possible for patients who underwent associated diaphragm resection (4 studies, n = 1,246 patients),18192931 associated vascular resection (2 studies, n = 195 patients),3032 and other MLRs (3 studies, n = 345 patients).172022
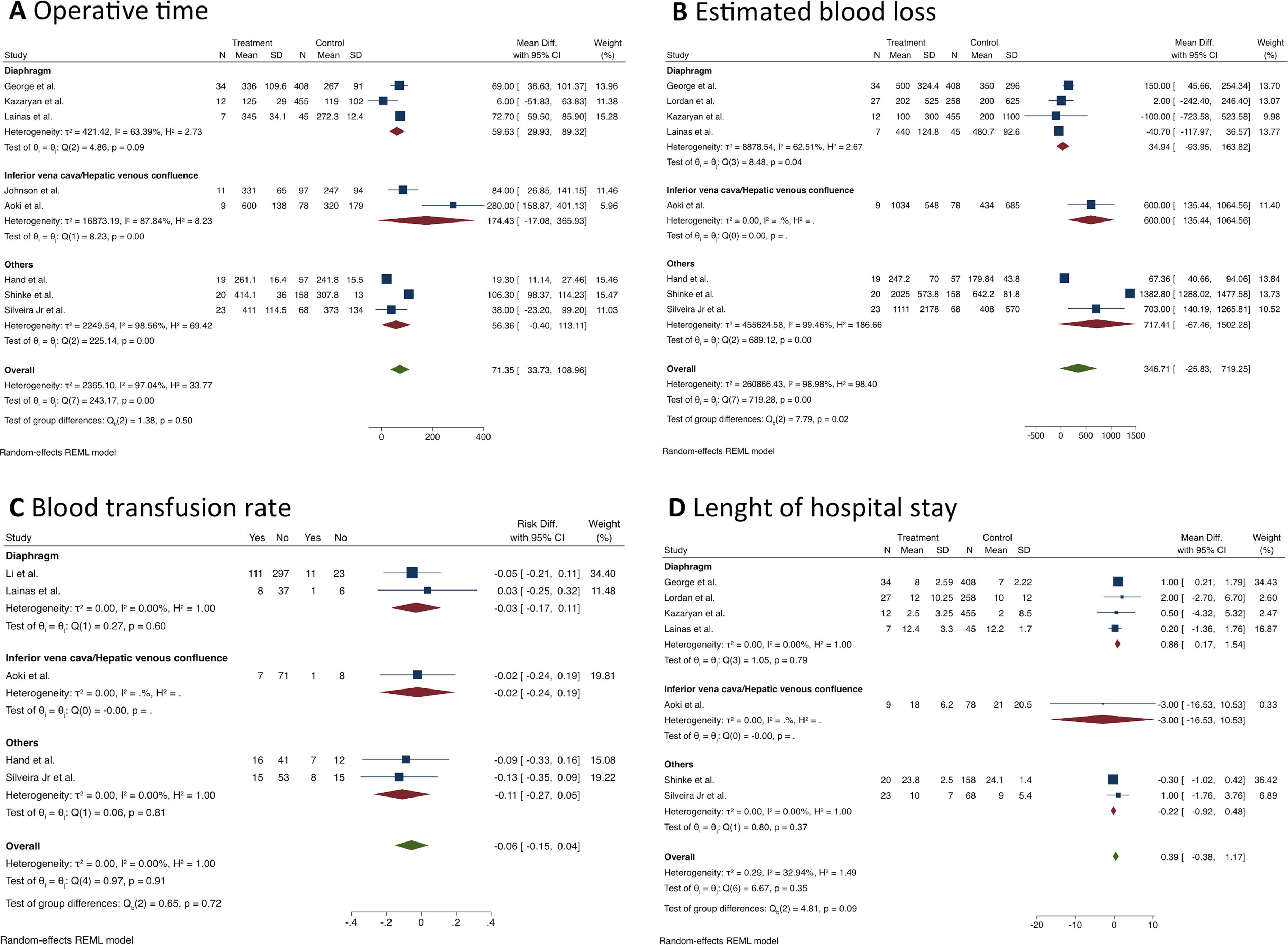
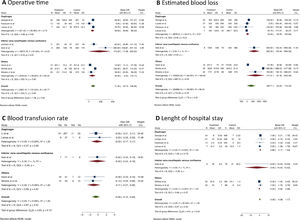
Short-term outcomesPatients who underwent MLR had longer operative times than standard hepatectomy (MD = 71.4 min; 95% CI 33.7 to 109; I2 = 97%; 8 studies; 1,501 patients; the certainty of evidence: low). The same finding was observed in the diaphragm resection subgroup (MD = 59.6 min; 95% CI 30.0‒89.4; I2 = 63.4%, Fig. 2A).
Estimated blood loss was assessed in 8 studies (n = 1,678 patients) (Fig. 2B), and the pooled analysis showed no difference between the groups (MD = 346.7 mL; 95% CI -25.8 to 719.3; I2 = 99%; certainty of evidence: very low). Similarly, no difference was found in the blood transfusion rate (RD = 0.06; 95% CI -0.04 to 0.15; I2 = 0%; certainty of evidence: very low, Fig. 2C).
Seven articles (n = 1,602 patients) reported results concerning the length of hospital stay (Fig. 2D), and no significant difference was found between the groups (MD = 0.39 days; 95% CI -0.38 to 1.17; I2 = 33%; certainty of evidence: moderate). However, MLR was associated with a longer hospital stay in the subgroup of patients who underwent diaphragm resection (MD = 0.86 days; 95% CI 0.17 to 1.54; I2 = 0%).
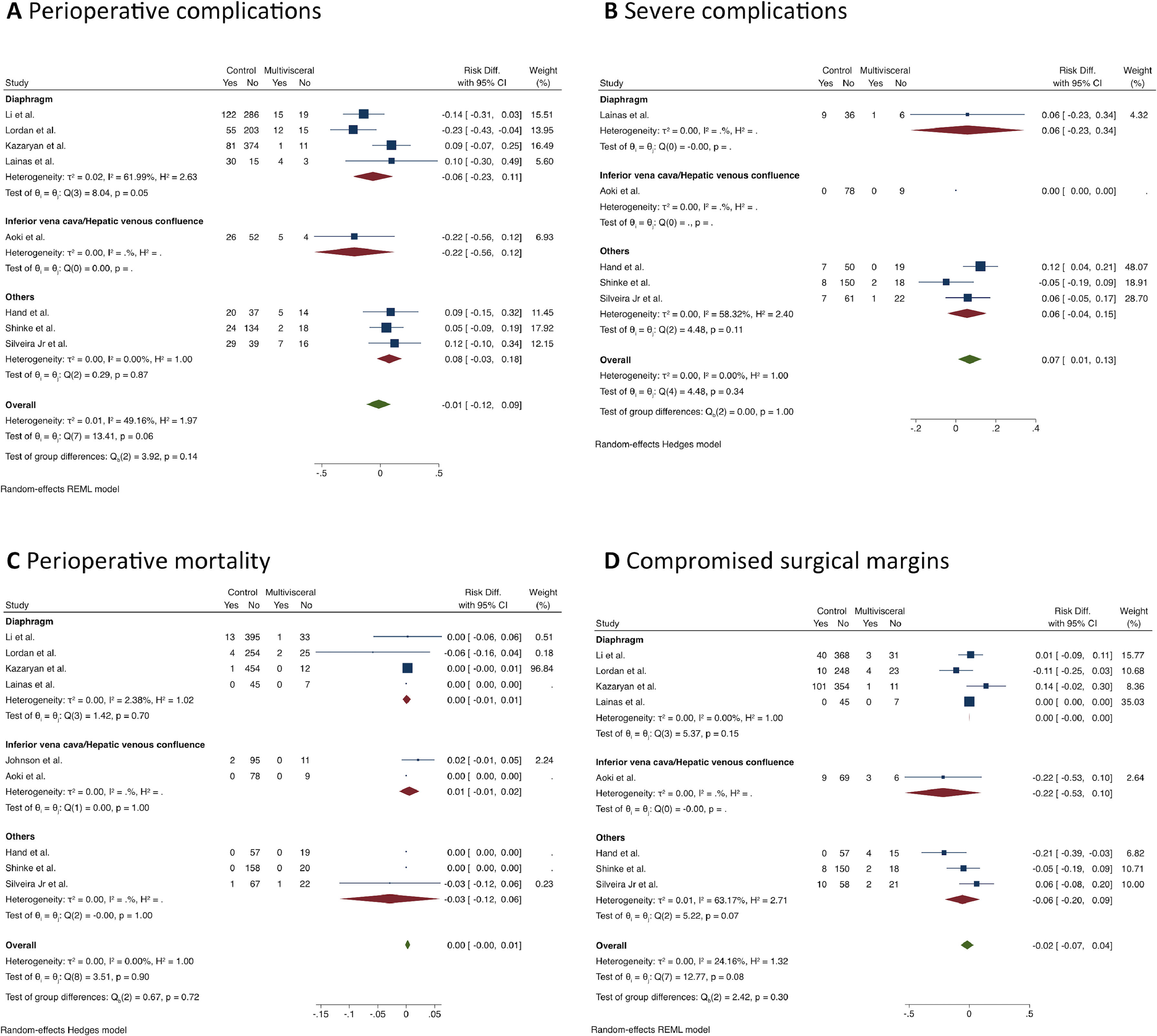
MLR was not associated with a higher risk for postoperative complications (RD = -0.01; 95% CI -0.12 to 0.09; I2 = 30%; 8 studies; 1,678 patients; certainty of evidence: moderate). However, analysis of the perioperative complications according to the Clavien-Dindo classification indicated that the MLR group had a higher rate of major complications (Grade III‒IV) (RD = 0.07; 95% CI 0.01 to 0.13; I2 = 0%; 5 studies, 484 patients; the certainty of evidence: moderate), but no differences were found in the subgroup analysis.
The reported perioperative mortality ranged from 0 to 7.4% in the MLR group and from 0 to 3% in the standard hepatectomy group (9 studies, n = 1,786 patients) with no difference between the groups (RD = 0.00; 95% CI -0.00 to 0.01; I2 = 0%; the certainty of evidence: moderate).
The frequency of compromised margins was also similar between the groups (RD = 0.02; 95% CI -0.04 to 0.07; I2 = 24%; 8 studies, 1,768 patients; the certainty of evidence: moderate), (Fig. 3).
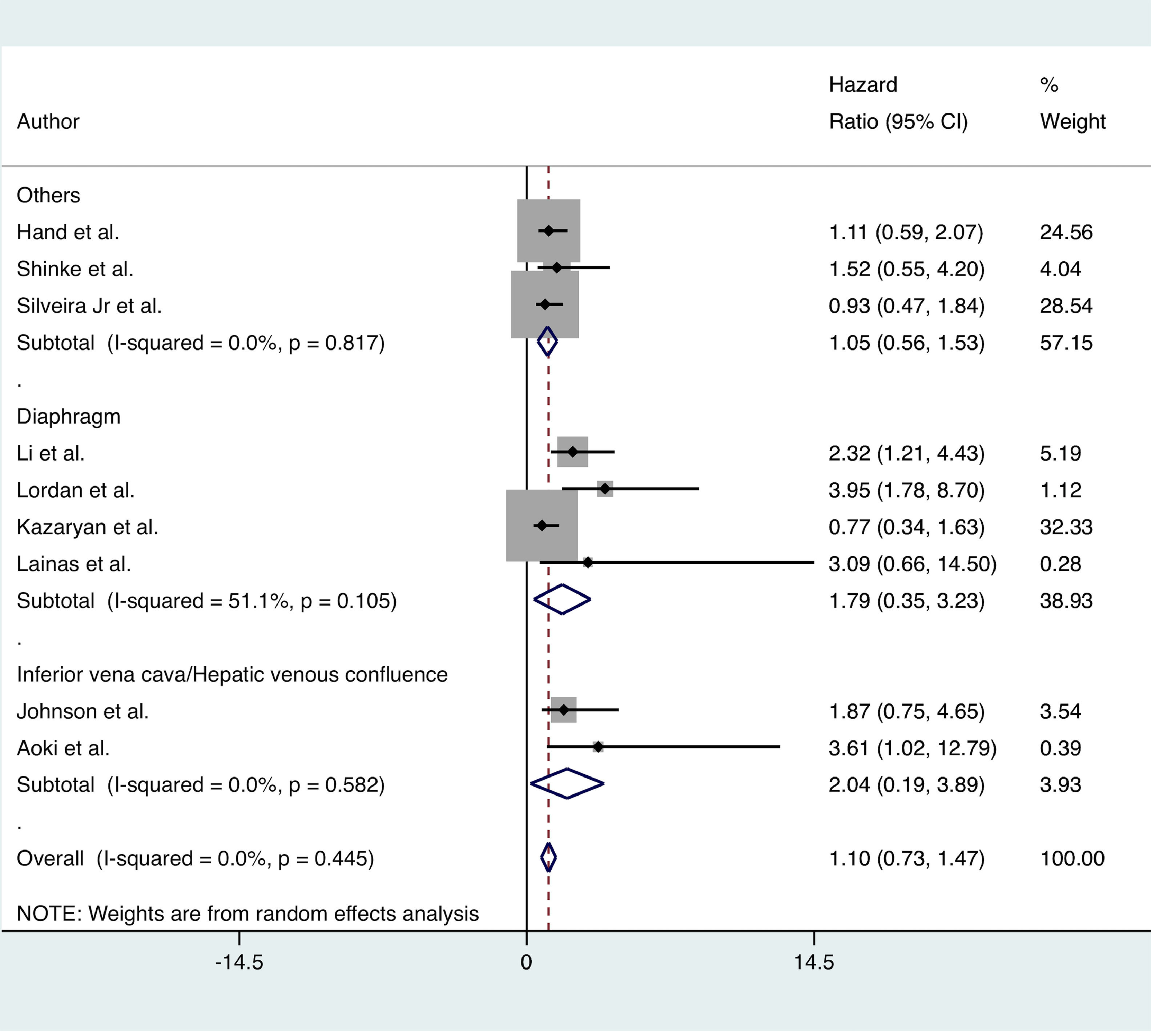
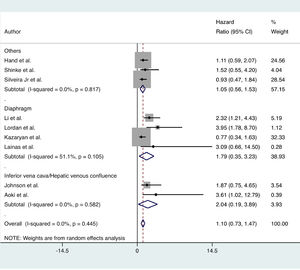
Long-term outcomesNo significant difference in OS was found between the MLR and standard hepatectomy groups (HR = 1.10; 95% CI 0.73 to 1.47; I2 = 0%; 9 certainties of evidence: moderate). Subgroup analysis showed similar results (Fig. 4).
DiscussionResectability of liver tumors is an evolving concept based on the possibility of radical resection of all tumor burdens with clear surgical margins. Therefore, MLR may potentially provide curative treatment for primary liver neoplasms, liver metastases, and tumors from other sites with a contiguous invasion of the liver.17,33-35
The short- and long-term outcomes of multivisceral resection have been studied for other gastrointestinal tumors, including colon, oesophageal, stomach, and pancreatic tumors.36–38 In a study from the present study's center, Dias et al.36 showed that multivisceral resection for gastric cancer is associated with higher perioperative complications (53.2% vs. 31.1%; p = 0.002) and shorter 5-year OS and DFS (55.4% vs. 71.5% [p < 0.001]; 51% vs. 77.8%; [p < 0.001], respectively) compared to a standard gastrectomy. Similarly, Petrucciani et al.,38 in a recent meta-analysis, showed that multivisceral pancreatic resection is associated with higher morbidity (56%‒69% vs. 37%‒50%) and mortality rates (10% vs. 4%) compared to a standard pancreatectomy.
MLR is still under debate because few studies have evaluated the impact of MLR on the outcomes of patients with malignant liver tumors. Although few studies have not found an impact of MLR on short- or long-term outcomes,1733 other studies have reported a negative impact of MLR on perioperative results.2239 Using a large database from the American College of Surgeons, Li et al.39 compared patients who underwent standard hepatectomy vs. en bloc hepatic and diaphragm resection due to several types of liver tumors, and they reported that the need for concomitant diaphragm resection is associated with a longer operative time, higher transfusion rate, longer length of hospital stay, higher overall morbidity, and higher frequency of major complications. Similarly, a recent matched case-control study (1:2) from the present group has reported that patients who undergo MLR have a longer operative time (430 vs. 360 min, p = 0.005), higher estimated blood loss (600 vs. 400 mL; p = 0.011), longer hospital stay (8 vs. 7 days; p = 0.003), and higher perioperative mortality (9.4% vs. 1.9%, p = 0.042). Importantly, the authors observed a higher density of deaths in the early time period after the resection, suggesting that the cumulative experience and improvements in perioperative care can decrease the mortality risk following MLR. Moreover, MLR does not negatively affect long-term outcomes.22 Therefore, an extended resection requires additional attention to postoperative complications and mortality, especially in the early time period after the resection; however, MLR may offer a valuable option of curative treatment for selected patients with locally advanced liver neoplasms.
The treatment of CRLM has largely evolved over the last decades, and it is currently based on the combination of modern systemic chemotherapy regimens and radical resection of liver lesions.7940 The OS rates of patients with CRLM who underwent curative-intent hepatectomy have increased, reaching 40% to 60% at 5 years.689 In contrast, for patients with unresectable CRLM, the median OS is 18 to 36 months with palliative chemotherapy regimens.4142 Negative surgical margins are associated with longer survival rates and a lower risk of local recurrence in patients with CRLM.111215 Based on these premises, when a locally advanced CRLM involves an adjacent organ, liver resection combined with resection of the involved adjacent organ is required for oncologic curative resection.
However, the impact of MLR on patients with CLRM is still under debate due to several limitations of the available studies. The first is the rarity of these procedures even in high-volume referral centers. Hand et al.17 found 19 (3.6%) patients who underwent MLR out of 523 patients operated on for CRLM between 2005 and 2015. In the present center, the authors found 68 (11.2%) cases of MLR out of 609 patients operated on for CRLM over a 12-year period.22 Therefore, one of the major concerns about the studies addressing this issue is the small underpowered sample size. Another limitation is the lack of a standard definition of MLR. For this reason, the authors handled this potential bias using a clear definition of MLR derived from the definition used for multivisceral pancreatic surgery.3843 Thus, MLR was defined as hepatectomy with en bloc resection of at least one adjacent organ or structure not usually removed in a standard procedure due to direct invasion by the liver tumor.22 Based on this definition, it is important to highlight that simultaneous resection of CRLM and the primary colorectal tumor were not considered an MLR.
Applying these criteria, the authors found only 9 comparative studies that assessed the outcomes of MLR in patients with locally advanced CRLM. Pooled analysis showed that MLR is associated with longer operative times, which is in line with other studies.1730 Aoki et al.30 showed that patients who undergo MLR due to IVC or hepatic venous confluent invasion required almost double the time for resection compared to patients who undergo a standard hepatectomy (600 vs. 320 minutes; p < 0.001).
Despite technical difficulties, no differences in terms of estimated blood loss or transfusion rate were observed. Moreover, no increase in the length of hospital stay was found, except in the subgroup of patients with associated diaphragmatic resection. Other researchers who exclusively studied combined liver and diaphragmatic resections have reported conflicting results.1939
Some studies have shown an increase in the perioperative complication rate in MLR; however, most of these studies included several different aetiologies in the same group.2234 Conversely, the present meta-analysis demonstrated that MLR did not increase the overall morbidity in patients with CRLM, which agreed with Li et al.,19 who compared patients with CRLM who underwent hepatectomy and diaphragmatic resection vs. standard hepatectomies and did not find a significant difference in terms of perioperative morbidity (44.1% vs. 29.9%; p = 0.085). Similarly, in a matched case-control study (1:2), Hand et al.17 compared patients with CRLM who underwent MLR to those who underwent isolated hepatectomy and found no increase in perioperative complication rates (26.3% vs. 35%, p = 0.90). Importantly, the authors found an absolute increment of 7% in postoperative major complications. This is an interesting finding because most of the available studies did not directly assess this specific endpoint.
No significant difference in postoperative 30-day mortality was found. None of the included studies showed an increase in perioperative mortality, reinforcing the safety of MLR in patients with CRLM.
Regarding oncological outcomes, no difference in the frequency of compromised surgical margins was found between the group, supporting the use of MLR because it offers a similar rate of R0 resections for patients with locally advanced CRLM. In the previous study, the authors found similar rates of negative resection margins in patients with CRLM who underwent MLR compared to those with underwent an isolated hepatectomy (91% vs. 82.8%, p = 0.723).22
The OS rate was similar between the groups, indicating that MLR may offer a unique and valuable option for potentially curative treatment of locally advanced CRLM. Similarly, Shinke et al.20 reported similar OS for patients who underwent MLR or standard hepatectomy for CRLM in a nonmatched comparative study. Recently, a matched cohort analysis study has also reported no significant difference in the 1-, 3-, and 5-year OS rates following multivisceral resection or standard hepatectomy (75% vs. 82.1%, 56.6% vs. 53.4%, and 25.7% vs. 30.3%, respectively; p = 0.78).17
The present meta-analysis had several limitations. First, there is a lack of a clear definition of MLR. Thus, the present study was designed to minimize this bias using a clear definition of MLR, excluding cases of non-contiguous resection and hilar resection.22 Other limitations included the small number of available studies and the observational design. Despite these drawbacks, the present study is the first meta-analysis to evaluate the short- and long-term results of MLR in patients with CRLM, including a significant number of patients in comparison groups. Therefore, the present findings are the best available because RCTs are still lacking. However, the present findings should be confirmed by larger well-designed studies.
ConclusionIn conclusion, MLR is a safe and feasible procedure but has a higher risk of major perioperative complications. MLR does not negatively affect long-term outcomes, indicating that an extended resection is a valuable option for potentially curative treatment for patients with locally advanced CRLM.
Data availability statementThe datasets generated during and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Ethics approval statementNot applicable.
Patient consent statementNot applicable.
Permission to reproduce material from other sourcesNot applicable.
Authors’ contributionsSérgio Silveira Júnior, data curation; Francisco Tustumi, formal analysis; Daniel de Paiva Magalhães, investigation; Vagner Birk Jeismann, methodology; Gilton Marques Fonseca, writing ‒ original draft; Jaime Arthur Pirola Kruger, writing ‒ review and editing; Fabricio Ferreira Coelho, conceptualization, supervision, and validation; Paulo Herman, conceptualization, validation, and data curation
Funding statementThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.