Laparoscopic procedures have usually been employed as an alternative to open surgery for the management of several urologic problems.1 Laparoscopic ureteroneocystostomy is rarely reported despite the description of different techniques.2-7 Ramalingam et al. described a 3-case series of successful laparoscopic Boari flap ureteral reimplantations.8 Gao el al. also described a 3-case series of laparoscopically ureteroplasties for the treatment of congenital obstructive megaureter with good results.9 Only one article considers ureteral tailoring and reimplantation, combining laparoscopic and conventional surgery to create an antireflux mechanism.10
We report, to our knowledge, the first case of laparoscopic ureteral reimplantation with intracorporeal tailoring for an obstructive megaureter in adults.
Case descriptionA 60-year-old male patient with hypertension, diabetes, dyslipidemia and chronic renal insufficiency not requiring dialysis (serum creatinine of 3,5 mg/dL) was diagnosed with severe left ureterohydronephrosis during a screening sonography. Voiding cystourethrogram revealed no vesico-ureteral reflux. Renal dynamic scan showed a functioning left kidney with a split function of 28%. Computed tomography scan demonstrated a marked dilation of the left pyelocaliceal system with reduced thickness of the renal parenchyma associated with a primary megaureter. The right kidney was anatomically and functionally preserved (Figure 1).
The patient was submitted to laparoscopic ureteral reimplantation with intracorporeal tailoring of the distal ureter. The patient was initially positioned supine for intravenous access, general anesthesia, bladder catheterization and orogastric tube placement. He was then positioned in a 30-degree left lateral decubitus; two supportive rolls were placed under his back and pressure points were padded. The patient was taped in position so that his arms, legs and abdomen remained securely in place. The table was then bended as required to assist with gravity retraction of the bowel.
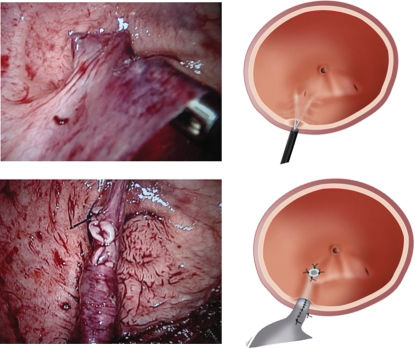
The first trocar (11 mm camera port) was inserted in the lower umbilical lip; two other trocars of 5 and 10 mm were located in the mid clavicular line on the left and right sides for the surgical instruments. A fourth 5 mm port was placed on the right anterior axillary line, immediately above the iliac crest (Figure 2).
Schematic drawing of the arrangement of trocars. The primary trocar (11 mm camera port) was inserted in the lower umbilical lip; two other trocars of 5 and 10 mm were located in the mid clavicular line on the left and right sides for the surgeon instruments. A fourth 5mm port was placed for the assistant on the right anterior axillary line, immediately above the iliac crest.
The sigmoid was reflected medially and the dilated distal ureter was easily identified. To facilitate its dissection down to the bladder, the ureter was emptied with an 18-G laparoscopic needle. A narrowed 10 mm distal segment of the ureter was identified, ligated at its bladder insertion and ressected. The ureter was longitudinally incised in a 6 cm extension over a 12-F plastic urethral catheter and intracorporeally tailored using a 4-zero polyglactin running suture (Figure 3).
The bladder was then filled with saline and longitudinally opened in the left posterolateral aspect. A 2.5 cm submucosal tunnel was created with blunt dissection at the lowest part of the bladder incision (Figure 4). The ureter was positioned through the submucosal tunnel and a mucosa-to-mucosa ureterovesical anastomosis was completed with 4-zero polyglactin interrupted sutures. The first stitch comprised the detrusor layer in order to avoid ureteral retraction. A 6Fr Double-J stent was introduced in substitution of the 12F catheter. A running single layer suture with 2-zero polyglactin closed the bladder. An extravesical attachment stitch between detrusor and ureteral adventitia was done with 4-zero polyglactin.
The operative time was 210 minutes. Blood loss was minimal. A left tubular vacuum drain was left in the patient for 48 hours. The Foley catheter was removed on the fourth postoperative day and the patient discharged from the hospital. The Double-J stent was left in the patient for four weeks. Histopathologic analysis of the distal ureter revealed a chronic ureteral inflammation with fibrosis between muscle fibers.
Postoperative course was uneventful and the DTPA-99mTc. DTPA (Diethylenetriaminepentaacetic acid) radioisotopic study four months after surgery demonstrated no ureteral obstruction and an improved left renal function. The voiding cystourethrogram showed no vesico-ureteral reflux on both sides.
DISCUSSIONAlthough open surgery currently remains the gold standard for lower urinary tract reconstruction, all operative steps can be duplicated laparoscopically.1,7,11 There are several techniques available for ureteral reimplantation, with success rates greater than 92%.1,6 Nevertheless, some of these procedures have not been widely accepted when done laparoscopically.3
In ureteral reimplantation of a primary megaureter, the ureteral diameter should be reduced from 1/5 to 1/3 of the submucosal length in order to obtain an adequate antireflux mechanism. Some authors have described a technique of extracorporeal tailoring for megaureter in order to allow laparoscopic extravesical transperitoneal antireflux ureteral reimplantation.10,12 Duplicating open techniques, we tailored the megaureter laparoscopicaly before its antireflux reimplantation.
A 12-F catheter was used to establish ureteral diameter. One of the most important steps of this procedure is ureteral stabilization. It facilitates tailoring and suturing over the plastic catheter, as well as the correct positioning of the ureter inside the submucosal tunnel before bladder anastomosis. In order to stabilize the ureter, we used laparoscopic forceps and a distal ureteral stitch to fixate the catheter. The correct ureteral width could then be easily obtained and the remaining ureter sewn over the catheter.
Opening the bladder wall longitudinally down to its base facilitated the dissection of the submucosal path and avoided ureteral kinks after reimplantation. In addition, the bladder base is the recommended area for ureteral reimplantation as it is more stable and may allow easier identification of the ureteral orifice in case a stent is needed post-operatively.
The surgery was done in an adult patient and may be considered an alternative to open surgery. It facilitated correct positioning of the neoureteral orifice inside the bladder, which is harder to reach in this population because of its pelvic situation. Its feasibility should be also confirmed in pediatric patients and greater investigation is required to evaluate long-term efficacy.