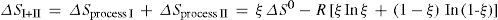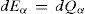The internal energy minimum as a criterion for equilibrium is here discussed in reference to a chemical reaction. That a non-isolated system at constant entropy and volume heads for a state of minimum internal energy is here shown via a thermodynamic analysis of the para-ortho isomerization reaction of hydrogen. The analysis brings forward the equations for the heat flow rate and temperature decreasing regime the reaction system has to comply with if a constant entropy path is to be accessed.
El mínimo de energía interna como criterio de equilibrio es aquí discutido con relación a una reacción química. El que un sistema no aislado, a entropía y volumen constantes, tienda a un estado de energía interna mínima se muestra aquí a través de un análisis termodinámico de la reacción de isomerización orto-para del hidrógeno. El análisis permite identificar las ecuaciones para los regímenes de flujo de calor y descenso de temperatura a los que debe someterse el sistema de reacción a efecto de acceder a una trayectoria evolutiva a entropía constante.
Classroom discussions on spontaneity and equilibrium commonly revolve around conditions of constant temperature and pressure, and constant temperature and volume, with little attention, if any, devoted to conditions involving a constant entropy restriction. That our students of thermodynamics deserve a complete as possible exposure to its fundamental concepts is beyond discussion. The accomplishment of this objective demands, however, an increased availability in the published literature of pertinent discussions that might be used to that end. Even if an excellent discussion could be found illustrating the constant entropy path for gas expansions (Velasco and Fernandez, 2002), apparently none is available applying this criterion to a chemical reaction, a most important subject indeed for chemistry students. It is with these considerations as a basis that we offer here a discussion on the thermodynamic conditions to be satisfied for the constant entropy evolution of the chemical reaction shown below. Now, even if as Emanuel (1987, p. 66) correctly points out, the statement ‘constant entropy’ can be associated to any process with identical entropy values for its initial and final conditions, here however, that statement is taken to mean constant entropy all along the path connecting those conditions
1.2Thermodynamic backgroundThe equilibrium state accessed by reaction (1) at temperature T is characterized by the thermodynamic equilibrium constant (K), defined as the ratio of the equilibrium activities (a) of ortho-hydrogen and para-hydrogen, K=aortho/apara. These activities, in essence non-ideality corrected equilibrium partial pressures or concentrations (Maron and Prutton, 1965, p. 203), make of K a true equilibrium constant, dependent only on temperature (Maron and Prutton, 1965, p. 231). In the case of gas-phase reactions taking place at moderate pressures and temperatures — where the assumption of ideal gas behavior is reasonable — no significant error is introduced in replacing activities by partial pressures (p) (Maron and Prutton, 1965, p. 205; Bevan Ott and Boerio-Goates, 2000, p. 438). Since, as will be seen below, this is the case for reaction (1), we can then write K in the following form: K = portho/ppara. A simple application of the ideal gas law can be used to reexpress this last equation as the ratio of equilibrium weight percentages (w) of the indicated species, i.e. K = wortho/wpara. Under the assumption of ideal gas behavior for the reacting species, the three different expressions for K, previously written, are equivalent.
K can also be defined in terms of reaction's (1) associated standard Gibbs free energy change (ΔG0) at the same temperature, ΔG0 = -RT ln K (Bevan Ott and Boerio-Goates, 2000, p. 437). This equation can be rewritten in terms of the standard enthalpy and entropy changes via the defining equation for the Gibbs free energy, G = H -TS, as follows: ΔG0 = ΔH0 -TΔS0 = -RT ln K.
Under the assumption of ideality, allowing the identification of K with the equilibrium weight percentage ratio, the following is true: ΔH0−TΔS0 = −RT Inwortho/wpara
The effect of temperature on K can be obtained by differentiating equation ΔG0 = -RT ln K with respect to temperature. Performance of this operation, combined with the fact that d(ΔG0 /T)/dT =-ΔH0/T2, leads to the following expression for the temperature variation of the equilibrium constant: d ln K /dT = ΔH0/RT2 (Bevan Ott and Boerio-Goates, 2000, p. 446).
Reaction (1) was chosen for this study because of its equilibrium constant having practically the same magnitude in the temperature interval 300 K–600 K. This temperature independence of the equilibrium constant allows us to conclude, in light of that expressed in the above written equation for the temperature variation of K, that in this temperature interval ΔH0 = 0. Introduction of this fact in the previously written equation relating ΔH0, ΔS0, and K, leads, in turn, to the following equation: TΔS0 = RT Inwortho/wpara, valid for ortho para ûú reaction (1) under the assumptions of ideality and temperature independence of K previously discussed. That this is indeed the case is shown in section 2.3.
1.3Internal energy minimum as equilibrium criterionThe entropy maximum principle as a criterion of equilibrium has been re-expressed through a number of thermodynamic functions which even if less fundamental and less general than the entropy law, are of more practical convenience in the study of some concrete problems (Lewis and Randall, 1961, p. 138). The Gibbs (G), and Helmholtz (A) free energy functions are among these alternative functions. The criterion of equilibrium for systems evolving at constant temperature and pressure is that G has reached its minimum possible value (Denbigh, 1968, p. 83). For those evolving at constant temperature and volume the criterion of equilibrium is that A has reached its minimum possible value (Denbigh, 1968, p. 82). Next to these, we find the minimum possible value of the internal energy (E) acting as criterion for the equilibrium state of systems evolving at constant entropy and volume. This lesser known criterion (Denbigh, 1968, p. 83; Richet, 2001, p. 35) is to be here discussed in reference to reaction (1). The essence of this discussion centers on the fact that if the reaction system is to be able to evolve at constant entropy, a way has to be found to transfer outside the reaction system the entropy increase associated with the reaction process. As will be seen below, this is here accomplished by coupling the reaction system with a cold reservoir whose function is to extract heat from the reaction system — and thus diminish its entropy — at precisely the same rate that entropy is created by the reaction. It is through the coupling of these two processes that the constant entropy path for reaction (1) will be accessed, a path leading the system to a state of equilibrium characterized by a minimum of its internal energy.
Still another equilibrium criterion can be found in the literature next to those already mentioned. This one associates a minimum value of the enthalpy function (H) with the state of equilibrium of systems evolving at constant entropy and pressure. Chemistry oriented exemplifications of this principle suitable for classroom presentations are also scarce or non-existent.
2The isomerization of hydrogen2.1The key assumptionThe work of Woolley et al. (1948, pp. 379-475) shows that in the interval 300 K–600 K, the equilibrium constant (K) for the para-ortho isomerization of hydrogen is, for all practical purposes, independent of temperature (the equilibrium composition up to 500 K can be read directly from Table 12 on p. 395. The equilibrium constant at 600 K can be calculated from the data in Table 4, p. 387). When expressed as the ratio of the percentages of ortho-hydrogen to para-hydrogen, the equilibrium constant changes from a value of 2.988 at 298.16 K to a value of 3.000 at 600 K. The percentage variation referred to the high temperature value amounts to 0.4%. In what follows, a number of considerations will be derived from what will be assumed to be a perfect temperature independence of K in the indicated temperature interval.
2.1(a). The fact that K ≠ f (T) leads to d ln K/dT = 0, and this, in turn, through equation d ln K/dT = ΔH0 /RT2, to the realization that ΔH0 = 0 in this temperature interval. This fact, combined with the assumption of ideal gas behavior for ortho-hydrogen and para-hydrogen — a reasonable assumption at the specified temperature interval and pressures near atmospheric — as well as with the fact that this reaction takes place with no change in total number of moles (Δn = 0), leads, through equation ΔH = ΔE +Δ(PV), properly modified for the case at hand as ΔH0 = ΔE0 +RTΔn, to the result ΔH0 = ΔE0. The already proven fact that in the case at hand we have that ΔH0 = 0 allows us to conclude that the following also holds: ΔE0 = 0.
2.1(b). From the data of Woolley et al. (1948, p. 387) we also learn that in the indicated temperature interval, the constant pressure heat capacities for ortho-hydrogen and para-hydrogen are not only practically identical, but also constant. From the ideal gas assumption introduced above it follows that their heat capacities at constant volume, assuming a relationship of the form Cv =Cp -R, will also be identical to one another and constant in the given temperature interval.
2.1(c). If as discussed in section 2.1(a): ΔH0 = 0 , then the thermodynamic expression connecting the equilibrium constant with the standard enthalpy and entropy changes, namely,In K = −ΔH0/RT + ΔS0/R, will reduce to: ln K = ΔS0/R . Inspection of this expression makes it obvious that the constancy of K assures the constancy of ΔS0 in the indicated temperature interval. As will be numerically corroborated below, this last expression also shows that in the indicated temperature interval, this reaction is solely driven by the entropy change of the reaction itself.
2.2The chemical reactionWith the previous considerations in mind, let us take a look at the situation depicted in Figure 1. In it use is made of the variable ξ the degree of advancement — through which the extent of the chemical reaction will be quantified. This parameter, with limit values of zero at the beginning of the reaction, and one at complete conversion (0 ≤ ξ ≤ 1), can for the case at hand be defined either as ξ = -Δnpara/1 or as ξ =Δnortho/1. In both instances Δn represents the mole number change of the indicated species. In the former expression the one in the denominator represents the initial number of moles of para-hydrogen in the reaction vessel, while in the latter the number of moles of ortho-hydrogen at complete conversion.
The para-ortho isomerization of hydrogen is shown here evolving from ξ = 0 to an arbitrary ξ, through the concatenation of processes I and II. Process I corresponds to the ξ fraction of a standard reaction, and process II to a mixing process. Process III represents, on the other hand, the cooling of the reaction mixture through which the constant entropy path is to be accessed. T* and P* represent, respectively, the temperature and pressure of the cold reaction mixture at ξ. Even if shown here taking place sequentially, the reaction and cooling processes are to take place simultaneously.
There, processes I and II depict, in a stepwise fashion, the advance of reaction (1) from an initial condition represented by 1 mole of pure para-hydrogen at 600 K and 1 atmosphere total pressure (ξ = 0), to a condition represented by the mixture of 1 - ξ moles of pure para-hydrogen and ξ moles of ortho-hydrogen, also at 600 K and 1 atmosphere total pressure. The 1 atmosphere condition is carried on from the standard state definition used by Woolley et al. (1948, p. 387). The reason behind the choice of 600 K as the initial temperature for reaction (1) will be explained below.
It has to be understood that the concatenation of processes shown in Figure 1 has been constructed to serve as an analytical tool in the development some of the thermodynamic arguments to be presented, and in no way implies that the reaction and cooling processes — to be described below — take place sequentially as shown. Quite the contrary, the chemical reaction described by the combination of processes I and II, and the cooling process, shown there as process III, are to take place simultaneously if the constant entropy evolution of the reaction system is to be possible. The description of steps I and II shown in Figure 1 is done in what follows.
Process I. This process corresponds to the reaction taking the system from pure para-H2 at 1 atmosphere and 600 K, to the indicated amounts of pure ortho-H2 and para-H2, each at 1 atmosphere and 600 K. This reaction is actually the ξ fraction of the standard reaction converting 1 mole of pure para-H2 into 1 mole of pure ortho-H2, both at 1 atmosphere and 600 K, and as such conveys an entropy change of:
According to that discussed in section 2.1(a), the enthalpy and internal energy changes associated to this process will be written as follows
Process II. Here the pure isomers in the amount and conditions shown as the final state of process I are brought together to produce what would be the actual reaction mixture at the given degree of advancement. The entropy change associated to this process corresponds to the entropy of mixing of the indicated amounts of para-H2 and ortho-H2, and as such, given by the following expression (Castellan, 1974, p. 231)
Due to the fact that the mixture here being formed is an ideal mixture, the enthalpy change for this step amounts to zero (Castellan, 1974, p. 231). A parallel argument to that advanced in section 2.1(a) allows us to conclude that here the internal energy of mixing is also zero. Therefore
By virtue of that expressed by equations (3), (4), and (6) it can be concluded that the reaction under consideration takes place without any thermal interaction with its surroundings, i.e. that no heat is at all exchanged between them as consequence of the occurrence of the chemical reaction, and consequently — as already mentioned — that this reaction is driven solely by entropic effects intrinsic to it. But if this is so then the universe of this reaction — the universe of steps I and II in Figure 1 — is the reaction system itself. That this is so is the matter of the following argument, through which the assumption introduced in section 2.1(a) is to be tested.
2.3Feasibility of the key assumptionFrom the data of Woolley et al. (1984, p. 387) for the para-H2 = ortho-H2 conversion, the value of ΔS0 = 2.2. cal/degree-mole, corresponding to 600 K, can be taken as representative for the previously indicated temperature interval. Being this so, the following expressions — produced via combination of equations (2) and (5) — can be written for the entropy change (ΔSI+II) of the indicated universe. The value for the ideal gas constant R has been taken as 1.99 cal/degree-mole, with ξ, as previously stated, representing the number of moles of ortho-H2.
A simple application of L’Hopital's rule to equation (8) produces, as should be expected, a value of ΔSI+II = 0 for the limit ξ → 0 . The behavior of ΔSI+II for larger values of ξ can be ascertained by taking the first derivative of equation (8) as follows
When this equation is put equal to zero, and the resulting expression solved for ξ , we identify an extremum for the entropy change of reaction (1) at ξ = 0.75. A simple application of the second derivative rule will confirm that this is a maximum, an entropy maximum, and as such it identifies the equilibrium condition for the reaction being considered. The equilibrium constant associated to the just determined equilibrium conversion for ortho-H2 can be obtained dividing it by the corresponding equilibrium conversion for para-H2, as follows: K = 0.75/(1-0.75.) = 3.0. The value obtained is similar to that already quoted of Woolley et al.
Further confirmation of the feasibility of our key assumption can be obtained calculating K via the equation introduced in section 2(c) and the previously quoted values for R, and ΔS0, as follows: K = exp (ΔS0/R) = exp(2.2/199) = 3.0. Again, the value produced this way is also similar to that also already quoted of Woolley et al. A similar analysis, leading to similar results, can be performed by the interested reader for the ortho - H2 → para - H2 reaction.
The previous analysis allows us to realize that the entropy change sustained by the universe for reaction (1), as given by equation (8) and represented by the concatenation of processes I and II of Figure 1, starts with a value of zero at ξ = 0 and increases monotonically until it reaches a maximum at an ortho-H2 conversion equal to ξ = 0.75. Being this so, it can only follow that any attempt to conduct this reaction through a constant entropy path will require a way to produce in the reaction system an entropy change of the same magnitude, but opposite sign, to the one quantified by equation (8). This effect will be brought about by coupling the unfolding of the chemical reaction — steps I and II — with the cooling of the reaction mixture, represented as process III in Figure 1. This cooling process will require the reaction mixture to be put in contact with a heat bath of low enough temperature to produce the desired effect. The particulars of this coupling are the matter of the following discussion.
3The constant entropy pathIn what follows, the concatenation of processes I, II and III, as graphically represented in Figure 1, will be designated as ‘the coupling’. Let us then start by agreeing that the indicated isomerization reaction is to take place at constant volume, and let us further define the reaction mixture as the system of interest (α). In thermal contact with the system we find a constant temperature bath (β). System and bath, combined, define the universe of the coupling. Let us further assume that the temperature of the bath is lower than that of the system. If this is so, a cooling process will take place alongside the chemical reaction. From the perspective of the system, this cooling process is an entropy reducing process. Thus, while the unfolding of the reaction increases the entropy of the system, the unfolding of the cooling process decreases it. These considerations allow us to realize that if we could couple the reaction and cooling processes in a way such that at every moment along their evolution every entropy increase produced by the reaction is met with an entropy decrease of the same magnitude produced by the cooling process, then our system of interest will be evolving along a constant entropy path. It has to be here recognized that even if a decreased rate of reaction is expected as a consequence of the cooling process, given the thermodynamic characteristics of the reaction system — discussed in sections 2(a) through 2(c) — as long as the cooling process is restricted to the temperature interval 300 K – 600 K, no effect whatsoever will be produced in its thermodynamics, as measured by its equilibrium conversion. An evolution under the considerations just advanced can be represented as follows:
In the previous equation, dSa, dSI+II and dSIII, respectively represent the net entropy change of the system, the entropy change associated to the unfolding of the chemical reaction, and that experienced by the reaction system due to the cooling process. These entropy changes, along that of the heat bath (dSβ) allows us to write the following expression for the entropy change of the universe of the coupling (dSu), i.e. the universe of processes I, II, and III, as follows
Upon substitution of equation (10) in (11) we learn that in the situation being considered, the entropy of the heat bath assumes the role of the entropy of the universe
It was stated above that from the perspective of the system the cooling process was an entropy reducing process. From the perspective of the heat bath, however, this is an entropy increasing process. The reason is simple. The bath is the one receiving the heat lost by the system. It is precisely upon the absorption of this heat by the heat bath that compliance with the dictate of the second law is produced, as in this situation equation (12) becomes:
Let us point out here that both, the system as well as the bath, are constant volume bodies incapable of any energy exchange in the form of work (we are assuming here that the only work interaction originally possible was of the PV kind). This consideration allows us, in turn, to write the following first law based expressions for the system and heat bath:
The fact that any heat lost by the system is necessarily heat gained by the bath can be represented as follows:
Combination of equations (14), (15), and (16) leads us to:
The fact shown in equation (15) stating that any heat exchanged by the heat bath is equal to the change of a function of state, allows us to write the following expression for the entropy change of the heat bath in terms of the internal energy decrease of the system
A comparison between equations (13) and (18) leads to the realization that at the conditions at hand the statement ‘an increase in the entropy of the universe’ becomes synonymous with the statement ‘a decrease in the internal energy of the system’. Actually the former is proportional to the latter. The proportionality constant being the inverse of the temperature of the bath. It is on reason of this proportionality that the spontaneity condition for a system evolving at constant entropy and volume can, alongside that expressed by equation (13), also be expressed as:
The message conveyed by equation (19) can be extended by saying that the equilibrium condition will correspond with a minimum in the internal energy of the system.
4The thermodynamic analysisAs previously stated, access to a constant entropy path for reaction (1) will be attempted by coupling processes I and II previously discussed, with a cooling of the reaction mixture, represented as process III in Figure 1. The role to be played by this process is described in what follows.
Process III. At any ξ in its advancement, the chemical reaction has an associated entropy increase quantified by equation (7). If a constant entropy path is to be accessed by this reaction, then at ξ the temperature of the reaction mixture has to have fallen to a value T* such that the entropy reduction produced by this cooling, precisely cancels the entropy increase associated to the reaction itself. The fact that no thermal interaction between the system and the bath is associated to processes I and II allows us to realize that the only heat to be removed from the system in process III is sensible heat, and due to this the entropy change sustained by the reaction mixture upon its cooling can be written as follows
Here Cυ stands for the constant volume heat capacity of the reaction mixture, T for the initial reaction temperature, and T * (ξ) — written like this to explicit its dependence on the degree of advancement — for the temperature the reaction mixture has to attain at every ξ in order to assure that ΔSIII will be equal in magnitude, but opposite in sign to that associated to processes I and II, as given by equation (7), i.e.
Fulfillment of this condition will produce — as shown in equation (10) — a combined value of zero for these two entropy changes, i.e.
It was through equation (22) that via a trial and error procedure, a temperature of 600 K was selected as the initial temperature for reaction (1). The selection criterion used was that of assuring the chemical reaction taking place within the 600 K – 300 K chosen for this study. The equations previously developed will be used in what follows to unveil the reaction mixture temperature and heat flow rate regimes required in order for equation (21) to be satisfied.
4.1The temperature decreasing regimeThe substitution of equations (7) and (20) in (21) leads to
Solving equation (23) for T * (ξ) produces the temperature decreasing regime the reaction mixture has to comply with in order for equation (21) to be satisfied and, consequently, in order to access a constant entropy path
The expression contained in the first equality of equation (24) comes from a combination of equations (20) and (21)
4.2The heat transfer regimeBeing T is the initial reaction temperature, and T*(ξ) the temperature of the reaction mixture at ξ , then the amount of heat lost by the system in its transit from ξ = 0 to ξ can be quantified as follows
In the previous equation the factor (1) has been introduced for unit consistency. It represents the constant total number of moles of reaction mixture. Related expressions will be subsequently written without this factor.
Substitution of equation (24) in (25) produces the expression quantifying the amount of heat to be removed from the reaction system as a function of ξ
4.3The heat flow rateThe heat flow rate that the reaction mixture has to experience in order to follow a constant entropy path comes via the first derivative of equation (26) with respect to ξ
If heat is removed of the reaction system at the rate mandated by equation (27), then the temperature decreasing regime embodied by equation (24) will follow, and the reaction system will be proceeding along a constant entropy path. In an experimental situation, equation (24) would provide a baseline against which the actual evolution of the system can be compared.
In what follows we will graphically display in Figure 2 the results of calculations carried on with some of the equations previously developed. In it ΔSβ was calculated as follows:
5A numerical exampleThe source of all the numerical data was the paper of Woolley et al. (1948, pp. 379-475). The system of units used in the said paper has been followed through. For the purpose of these calculations the magnitude of the equilibrium constant K = ξeq/1−ξeq was taken to be 3.0 in the 300 K – 600 temperature interval. Accordingly, the equilibrium conversion of ortho-H2 amounts to ξeq = 0.75. The value of ΔS0 = 2.2 cal/degree-mole, corresponding to 600 K, was taken as representative for the indicated temperature interval. Likewise, an average value of Cυ = 5.0. cal/degree-mol was selected. As previously stated, the ideal gas constant was taken as R = 1.99 cal/degree-mole.
Substitution of the appropriate values in equation (24) allowed us to calculate the final — and lowest — temperature of the reaction system along the cooling process. The value obtained was T*(ξequilibrium) = 345 K. This temperature, it should be realized, corresponds to the highest possible temperature for the heat bath, as then the system and heat bath would reach thermal equilibrium the moment the reaction reaches the condition of chemical equilibrium. For purposes of this illustration, this temperature was taken to be the constant temperature of the heat bath. As noted previously in the text, the initial temperature selected was T = 600 K.
For a given value of ξ, ΔSI+II is calculated through equation (8). The substitution of this value alongside those of T and Cυ in the expression formed in the first equality of equation (24), produces the T* corresponding to the given ξ . Substitution of this T*(ξ) in equation (20) produces ΔSIII . The addition of ΔSI+II and ΔSIII leads to ΔSα which, within the calculations uncertainty, should be equal to zero for all ξ. Finally, the values corresponding to ΔEα and ΔSβ at the given degree of advancement are calculated through equations (25) and (28).
It is evident from the figure that under the restrictions of constant entropy and volume imposed to the evolution of the reaction system, the equilibrium condition corresponds with a maximum in the entropy of the universe — here under the guise of the entropy of the heat bath, as well as with a minimum in the internal energy of the system.
6Final commentThe stated goal of this exercise was that of bringing to light the thermodynamic requirements associated to the constant entropy evolution of reaction (1). No attempt has been made to dwell into the practical aspects associated to actually carrying an experiment in this direction, nor into the thermodynamic conditions associated to the constant entropy evolution of the typical chemical reaction for which K is temperature dependent. It is true that in this regard reaction (1) is exceptional. However, in concurrence with C. E. Hecht (1967), we will have to recognize that more often than not, unusual, special, and even paradoxical situations serve well in illustrating the general concept.
Hopefully, this paper will prompt future discussions in these areas.