We report on learning progression research that tracks how students develop an understanding of matter and energy conservation as they pertain to the carbon-transforming processes of combustion, photosynthesis, cellular respiration, digestion, and biosynthesis. We find that typically only 10% of students in American high schools develop scientific explanations of these processes where they successfully conserve matter and energy (atoms in the inputs match those in the outputs and chemical energy is accurately associated with changes in chemical bonds). Students with confused explanations do not use the conservation laws to monitor their ideas. We present data that indicate that explicit instruction and consistent assessment on the use of the conservation laws as tools for understanding the carbon-transforming processes can advance students’ understanding.
Este artículo describe nuestras investigaciones respecto a las progresiones de aprendizaje que los estudiantes desarrollan al comprender tópicos de conservación de materia y energía, que se relacionan con procesos de transformación de carbono tales como combustión, fotosíntesis, respiración celular, digestión y biosíntesis. Nuestras investigaciones señalan que, en general, solo el 10% de los estudiantes de preparatoria de los Estados Unidos son capaces de explicar científicamente estos procesos utilizando las leyes de conservación de la materia y la energía en forma correcta, es decir, en que los átomos de los reactivos coinciden con los de los productos y la energía química se asocia correctamente a cambios en los enlaces químicos. En cambio, los estudiantes cuyas explicaciones son confusas no utilizan las leyes de la conservación de la materia y la energía para elaborar sus ideas. En este artículo presentamos datos que indican que la enseñanza explícita de las leyes de conservación de la materia y la energía y el uso de evaluaciones que sirvan como herramientas de aprendizaje, favorecen el avance y la progresión de los estudiantes al comprender los procesos de transformación de carbono.
We are interested in how students develop “increasingly sophisticated understandings” (Duschl, Schweingruber, & Shouse, 2007) of matter and energy conservation as they pertain to carbon-transforming processes. In particular, we are concerned with students’ thinking about photosynthesis, cellular respiration, digestion, biosynthesis, and combustion. The learning progressions presented here look at students’ growing understanding of how to distinguish matter from energy in these complex processes and trace changes in matter and energy in concert. We present the learning progression research and framework and examine their implications for instruction and assessment.
We focus on these processes because of their importance: They are responsible for growth and metabolism in all organisms, carbon cycling and energy flow in ecosystems, and over 90% of the energy that powers human economic activities. The global imbalance between the process that creates organic carbon — photosynthesis — and the processes that oxidize organic carbon — combustion and cellular respiration — is the primary cause of global climate change.
Findings from separate learning progressions on matter and energyLearning progressions on related topics have been published. Stevens, Delgado, and Krajcik (2010) developed a multi-dimensional learning progression for the nature of matter. In particular, they focused on “how students’ understanding of the models of atomic structure and the electrical forces that govern interactions at small scales develops from grades 7 to 14” (p. 690). Based on a previously developed learning progression for K-8 students’ understanding of atomic molecular theory (Smith et al., 2006) and a broad empirical sample, their learning progression describes students’ conceptions of atomic structure that range from atoms as spheres to the electron cloud model. For inter-atomic interactions, students’ conceptions ranged from an unspecified force that governs interaction to a definition of groups based on electron configuration. The authors suggest several instructional strategies, such as focusing on models and modeling, to support students’ understanding of atomic structure.
Neumann et al. (2013) report on an initial learning progression for energy based on existing curriculum, research on students’ understanding of energy concepts, and empirical evidence from students in grades 6, 8, and 10. They found that students generally progressed from understanding forms of energy, then energy transfer and degradation, and finally energy conservation. The authors do not suggest, however, that these conceptions are separate levels of understanding or that students progress linearly through the levels. Indeed, Neumann et al. explain that students seem to develop some understanding of energy transfer and transformation before developing a complete understanding of energy forms and sources. Therefore, the authors propose that “it is not wise for all possible forms (and sources) of energy to be covered in the curriculum before the concept of energy transfer and transformation is introduced” (pp. 184-185). As for energy conservation, the authors conclude that few students develop a full understanding of this concept and then only at the end of grade 10.
Although these studies are informative, they do not focus on the phenomena that we are most concerned about — carbon-transforming processes in living systems and human energy systems. Developing a learning progression for these processes is challenging because they are at once very familiar and very complex. Children of all ages are familiar with the macroscopic manifestations of these processes — including plant and animal growth, animal movement, decay, and combustion — and have developed explanations for these familiar phenomena. Learning about the complex chemical changes that drive these familiar processes is a long and difficult journey and the focus of our learning progression research.
Why we examine matter and energy in concertWe choose to examine students’ understanding of matter and energy in concert for a number of reasons. Although energy is not an object or a substance, it must be identified by its association with objects and substances or changes in objects or substances. In much of K-12 chemistry, the focus is on energy in the form of chemical potential energy associated with chemical bonds. In the narrower domain of carbon-transforming processes, we focus on chemical potential energy associated with systems containing oxygen and organic molecules that can be identified by their carbon-carbon and carbon-hydrogen bonds, compared to the lower energy bonds in carbon dioxide and water.
Matter and energy conservation as a crosscutting concept and constraintA second reason that we look at matter and energy in concert is that the conservation laws are powerful conceptual tools for understanding. In the United States, The Next Generation Science Standards (NGSS, NRC 2013) and The Framework for K12 Science Education (NRC, 2012) identify “energy and matter: flows, cycles, and conservation” as one of seven crosscutting concepts that students can use as organizational tools as they develop and check their growing understanding. “Tracking fluxes of energy and matter into, out of, and within systems helps one understand the systems’ possibilities and limitations” (NRC 2012, p. 84). Matter and energy conservation together provide an approach for making sense of new phenomena by identifying the changes in matter and transformations of energy, and accounting for all of the atoms and energy before and after the event. The conservation laws also provide a constraint on accounts of what is occurring. An explanation is not complete or accurate unless one can account for all of the matter and energy before and after the event. Scientists and students who can use the conservations laws in this way feel a sense of necessity that their explanations not violate the laws.
Below is a quote from a student who did not immediately know the source of the mass gained by radish seeds that received water and sunlight. However, he realized that he needed to account for each of the elements in the glucose that was produced by photosynthesis. Tracing matter was a tool that he used to generate an explanation. And then how this increase in mass bio-mass [radish seeds in light] occurred would obviously be not from water, so it had to be from something else like some sort of glucose or something like that… It [glucose] is made of C6H12O6 and so it needs the CO2 to make for the carbon and it has water that uses H for the water, too (Parker et al., 2012).
Because we are interested in students’ ability to use the conservation laws as schemata for understanding carbon-transforming processes, the learning progression we describe here is based on analysis of interviews of students’ and experts’ written responses to open-ended questions. The results reported here are based on interviews with 8 elementary, 22 middle school, and 26 high school students and written responses to open-ended questions by 481 elementary, 1001 middle, and 740 high school students (Jin and Anderson, 2012; Mohan, Chen, and Anderson, 2009). Students came from a variety of settings from several states. Interview and written questions were designed to minimize the need for knowledge of specific vocabulary. Tests and interviews included questions about everyday situations so that all students would have something to contribute. We looked for patterns in their responses and organized groups of similar responses by degree of sophistication. Longitudinal studies were used to see if individual students actually progress through the designated levels. We have done parallel work with undergraduates in introductory biology courses using interviews and essay and forced-choice questions (Parker et al., 2012; Wilson et al., 2006; Hartley et al., 2011).
The questions in our tests and interviews began with macroscopic processes that all students were familiar with — plant and animal growth, animal movement, decay, and combustion. We were especially interested whether students could trace matter and energy through these processes. We also asked follow-up questions that focused on students’ abilities to connect these macroscopic processes with atomic-molecular explanations — identifying hidden chemical changes — and with carbon cycling and energy flow in large-scale systems.
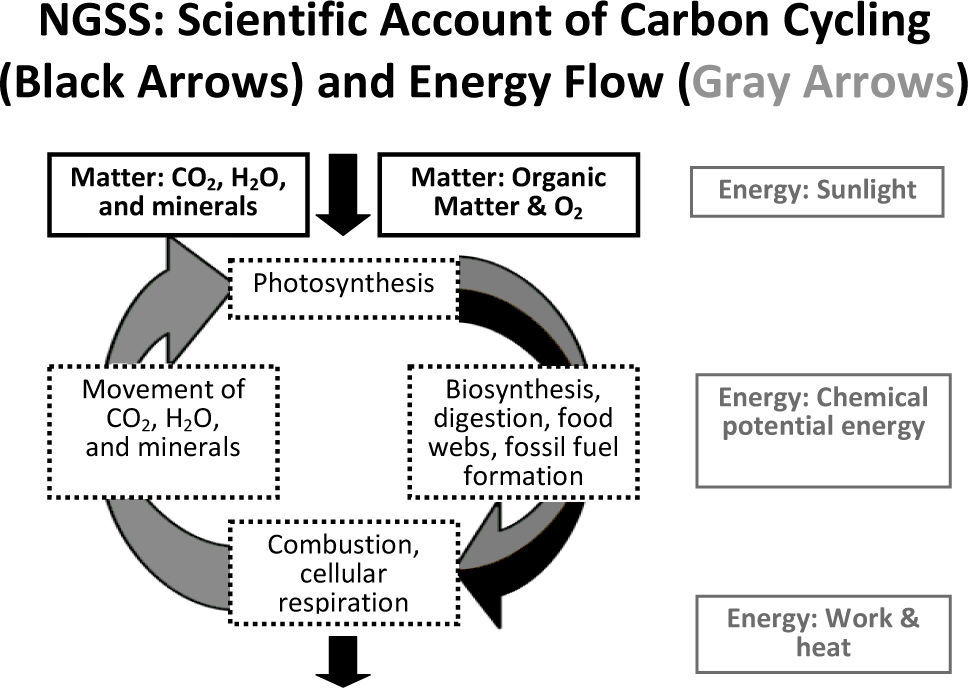
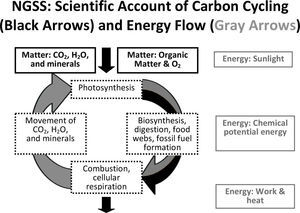
Scientific accounts of carbon-transforming processesFigure 1 represents the type of understanding that we would hope students have at the end of high school. In this diagram of ecological carbon cycling and energy flow, matter (represented by black text and arrows) cycles between living things and the atmosphere. Energy (represented by gray text and arrows) flows through ecosystems. Sunlight is transformed into chemical energy in biomolecules identi-fied by their C-C and C-H bonds and eventually in phosphate bonds in ATP and finally into work and heat which cannot be reused by living organisms.
Below are excerpts from interview transcripts from students who think in this way. Sabrina: It [decomposition] is cellular respiration. The carbon in glucose and the oxygen from air transform into CO2 and H2O. Eric: Yeah, the energy that the tree stored while it was alive through photosynthesis, that energy is now being released by various organisms eating and breaking apart those bonds that the tree had made and so that is releasing the older energy that the tree had stored.
Both Sabrina and Eric explain carbon-transforming processes in ways consistent with ngss and scientific accounts. They trace matter and energy across scales without confounding the two. Tat is, they explain macroscopic phenomena (in this case, decomposition) by describing matter and energy changes as the atomic level. Sabrina accounts for the elements carbon and oxygen in both inputs and outputs. Eric indicates that energy is not always associated with the same atoms. He associates energy with molecules that have reduced forms of carbon and hydrogen (C-C and C-H bonds) rather than oxidized forms (C-O or H-O bonds). We find that only about 10% of high school students typically give this type of scientific account of carbon-transforming processes (Mohan, Chen, & Anderson, 2009; Jin & Anderson, 2012).
Learning progression levelsThe previous section describes the scientific or highest level understanding that we hope most high school students will achieve. We designate this Level 4 understanding and categorize less sophisticated reasoning into Levels 1 through 3. Many younger students give accounts of the same processes described that differ greatly from the scientific or Level 4 accounts. In the following sections we describe the characteristics of the accounts that each level of student gives and illustrate these with multiple examples drawn from interviews.
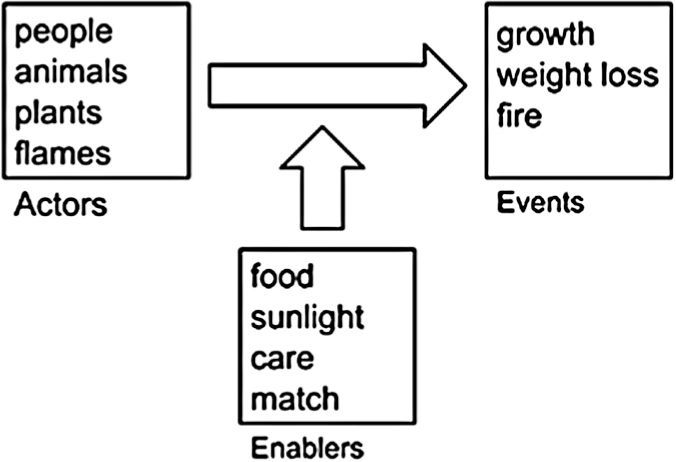
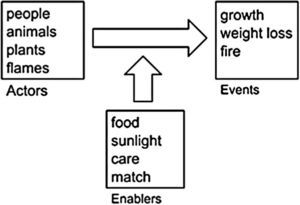
Level 1 accounts – stories with actors and enablersLevel 1 students are almost exclusively elementary or middle school students. However, their thinking sheds light on the origin of older students’ ideas. Figure 2 is a schematic of Level 1 thinking. More sophisticated students recognize that all materials, including the bodies of plants and animals, are made of microscopic subsystems and ultimately of atoms and molecules. Tough Level 1 students often know facts about atoms and molecules, those facts have no explanatory power for them. “Chemicals” for Level 1 students are esoteric materials that might enter our bodies through pollution or food additives, but certainly are not the basic materials that our bodies are made of.
Similarly, Level 1 students are familiar with “energy,” but energy for Level 1 students has little relationship with the sci-entific conception of energy. For phenomena involving living things, Level 1 students associate energy with life or vitality (“You need to sleep so that you will have plenty of energy.” “Running is good for you because it gives you more energy.”)
So rather than tracing matter and energy, Level 1 students focus on what they can see, and they interpret events in everyday language. They envision the events of the world as natural processes that occur when actors such as people, animals, plants, or even fames have what they need. These enablers can include materials (e.g., soil minerals for plant growth), energy sources (e.g., sunlight), causes (e.g., the match that starts a fire), or conditions (e.g., warmth or care). For Level 1 students, a good explanation tells how the en-ablers help the actors to achieve their purposes. They do not see that, “you are what you eat.” They think of food as the necessary enabler for life, growth, or energy, but not as a substance that becomes part of the eater or chemical potential energy. Thus materials may appear or disappear or the fate of materials may not be part of the story at all.
The following quotes are representative of the accounts of Level 1 students. Here Amber is explaining what happens to matter/food when a child runs. After the child eats the hamburger, it’s all energetic, so then he’ll want to run around…And it’s something that usually happens after you just ate something.
Thus for Amber the child is an actor and the hamburger is an enabler that the child uses to accomplish his purpose — running around. Energy is the “life force” that makes running around possible.
In response to a question about where the mass in a large tree comes from, Marcos explains: Water, sunlight and air. Water helps the tree to grow. It helps it to grow better. Because we need water, so do trees. The tree uses sunlight by helping it grow big and strong. Air helps the tree to grow because if it doesn’t have air, it will die. If we don’t have air, we will die.
Marcos’ explanation focuses on the tree as an actor that uses enablers (sunlight, water, air) to achieve its purpose — to “grow big and strong.” But he does not distinguish between the enabler that is an energy source (sunlight) and the en-ablers that are matter sources, nor does he trace the matter the tree is made of back to its origins. Ken describes what happens to gasoline when a car runs. Interviewer: Tell me more about gas. You said that gasoline helps the engine. What is in the gas that helps the engine? Ken: The gas helps the engine run so that way it’s basically the engine who gets it all, because that’s how it runs, and if you’re out of gasoline, you can’t usually move it anywhere.
For Ken the engine is an actor “who” gets the enabler — gasoline — to accomplish its purpose — to run.
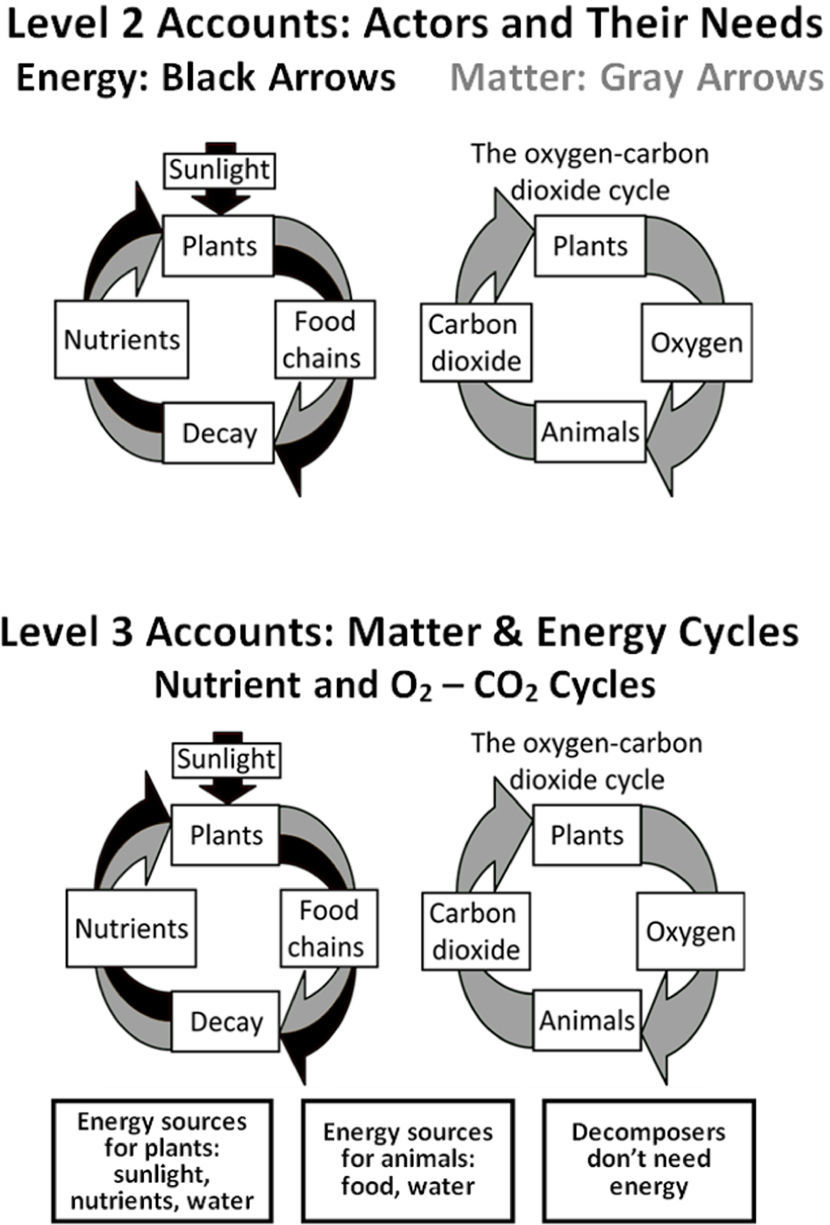
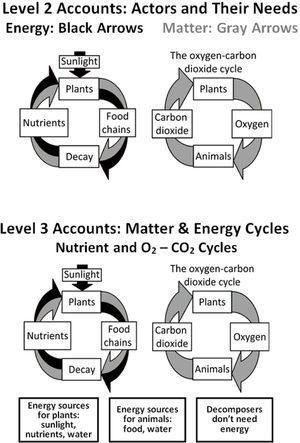
Level 2 accounts – more sophisticated storiesLevel 2 reasoning (Figure 3) is common in students of all ages, from elementary school up to and including college science majors. Level 2 students still tell stories about actors and enablers, but they include additional details which allow them to recognize the importance of subsystems (though still not atoms and molecules). In their stories, spe-cific processes have specific needs. The stories often include material inputs and outputs, but the inputs and outputs are restricted to what is visible and a few specific gases. Level 2 students recognize that organisms are made of cells and (sometimes) that cells are made of molecules, but they still are unable to connect life processes or combustion to chemical change — they do not think of the inputs as being transformed into the outputs.
The beginnings of matter-tracing strategies are apparent in Level 2 accounts, as illustrated in Figure 3. Some vague solid matter, often identified as “nutrients”, cycles between organisms. Thus these students identify a cycle where plants produce oxygen for animals and animals produce carbon dioxide for plants. Thus gases cycle separately from solids and liquids. Level 2 “cycles,” though, are primarily sequences of events rather than attempts to trace matter (i.e., more like life cycles than like scientific matter cycles). People and animals need food which they get either directly or indirectly from plants, and plants need nutrients which they get when living things decompose. Fire needs fuel.
The connections between inputs and outputs don’t follow scientific rules such as conservation of matter. In the examples below, Level 2 students identify soil, fertilizer, sweat, and ash as inputs or outputs. Atoms are not traced and materials may turn into energy. Food or fuel is seen as a physical necessity for some hidden process. Energy may be a ubiquitous enabler or connected with particular substances.
Teresa was asked to describe what happens to food when it is eaten: [The child] needs the energy from the cow meat in order to run.
She associates energy with the meat, but does not describe changes to it or its fate when eaten.
Nike can go farther with his explanation of the relationship between sunlight and food in plants. However, he does not talk about the atoms or the bonds in the substances he names and his description of sunlight’s role in photosynthesis makes it sound like a substance that can “mix” with other substances. Interviewer: Why do you think water, sunlight, carbon dioxide and soil have energy? What makes them have energy? Nike: Oh, atoms. Energy is like, we all have energy and it helps us move. And it goes through a change with — it goes through a change … Interviewer: So tell me more about where does the energy of sunlight go in relation to tree growth? Nike: The sunlight goes into the roots, or the leaves. And that helps create food with all the… I guess it mixes, I guess you could say, with water, soil, and carbon dioxide, and it all helps create the food for the tree in order for it to grow and get more weight.
John’s explanation of the fate of the chemical energy in gasoline shows a common problem had by students who do not conserve matter or energy – they do not know how to interpret the commonly used phrase, “it was used up.” Interviewer: So, where does the gasoline go? John: The gasoline is used up by all the parts. It’s also exhausted. It’s exhausted through the gas pipe or the exhaust pipe I mean. And it goes back into the air. Interviewer: Ok. So do you think the car needs the energy in order to move? John: Yes. The gasoline is their form of energy. Interviewer: Ok. So when gasoline is used up or becomes exhaust, where does the energy go? John: The energy goes with it into the air, back into the air. Interviewer: What form of energy is that? John: Hmmm.
Level 3 students (Figure 3) are mostly high school students (or older). They have a more microscopic view of the world that includes cellular processes such as respiration and photosynthesis, and they use many scientific vocabulary words. Rather than identifying actors and enablers, they are much better at identifying key subsystems (such as cells, molecules, and atoms), materials (such as glucose and other organic materials in addition to oxygen, carbon dioxide, and water), and forms of energy. They recognize that there are chemical explanations for combustion and life processes, and they work to produce those explanations.
However, their knowledge of chemical facts usually is not associated with a commitment to chemical principles such as conservation of matter and energy. These students attempt to identify the elements in some inputs and/or outputs. In addition, they add energy into their accounts of the food chain and combustion, but they make mistakes and therefore their accounts of cycles are very similar to those of Level 2 students (Figure 3). For example, energy may cycle with the carbon returning to plants in the nutrients in the soil (i.e., both energy and matter recycle), or energy may get used up and disappear. In general, Level 3 students trace matter and energy intermittently, inconsistently, inaccurately or incompletely. Level 3 students know about the laws of conservation of matter and energy, but they often give accounts that do not follow conservation rules. Matter-energy transformations (e.g., gasoline is converted to energy when it burns, fat is converted to energy when a person loses weight, plants convert sunlight into food) are common in Level 3 accounts. The quotes below give examples of Level 3 thinking.
Justin’s account of why a match gets lighter as it burns identifies a specific chemical output to explain the weight loss, but he does not associate this with an input. Justin: The match gets lighter because the match is getting smaller and the CO2 is leaving.
Richard’s account of what happens to gas in a car is “almost there.” He mentions bonds, but does not associate these directly with energy. He recognizes carbon dioxide as a product but does not trace the carbon back to gasoline as a carbon-containing reactant. Like many Level 3 accounts, Richard’s account includes a matter-energy conversion, suggesting that the energy in gasoline is converted to carbon dioxide. Richard: The gasoline is burned while it’s in the engine. And all the bonds in it are broken and rearranged. And then it goes out the exhaust into the atmosphere as carbon dioxide… Interviewer: So where does the energy initially in the gasoline go? Richard: It runs through the engine and then is converted to carbon dioxide.
We hope that the description of the levels of the learning progression conveys a sense of why so many students struggle to achieve the scientific understanding of carbon cycling as described in Figure 1: This understanding is a significant intellectual accomplishment, requiring students to develop new ways of interpreting familiar phenomena. In particular, students must learn to explain familiar macroscopic phenomena in terms of underlying chemical changes. One way to think about the nature of this accomplishment is by thinking about how students must change their thinking about how the processes are alike and different.
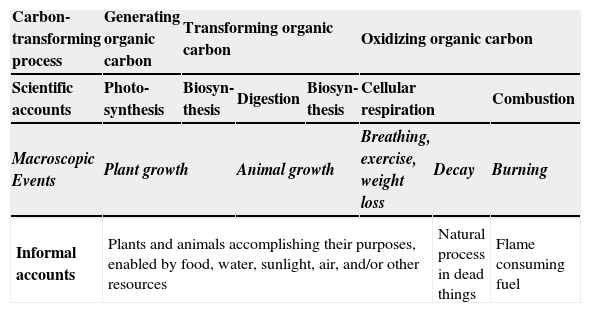
Table 1 (from Mohan, Chen, & Anderson, 2009) illustrates this point. The familiar processes that we asked students to explain are on the shaded row in the middle of the table. Lower-level students see the processes involving living plants and animals as all similar — driven by living actors and their enablers. For these learners, decay is quite different — something that happens naturally to dead things, and combustion is also different. Level 4 students, on the other hand, are able to classify the processes according to their underlying chemical changes, so these learners see quite different patterns in what is alike and different. Photosynthesis is unique as a process that creates organic materials out of inorganic matter. Plant and animal growth and food chains involve multiple transformations in organic matter. Tree processes that seem completely different to lower-level learners — animal movement, decay, and combustion — are seen by Level 4 students as all releasing energy by oxidation of organic matter.
Contrasting ways of grouping carbon-transforming processes.
| Carbon-transforming process | Generating organic carbon | Transforming organic carbon | Oxidizing organic carbon | ||||
|---|---|---|---|---|---|---|---|
| Scientific accounts | Photo-synthesis | Biosyn-thesis | Digestion | Biosyn-thesis | Cellular respiration | Combustion | |
| Macroscopic Events | Plant growth | Animal growth | Breathing, exercise, weight loss | Decay | Burning | ||
| Informal accounts | Plants and animals accomplishing their purposes, enabled by food, water, sunlight, air, and/or other resources | Natural process in dead things | Flame consuming fuel | ||||
In the learning progression research described here we focused on development of students’ ability to use matter and energy conservation laws to explain the carbon-transforming processes of photosynthesis, cellular respiration, decomposition, and combustion. Many of the aspects of this type of reasoning apply more broadly to general chemistry. For example, as in this work where a goal for students was recognizing the chemical basis of life, a goal of general chemistry is that students recognize the chemical basis of all materials and many familiar phenomena: we want students to be able to reason about systems and processes at different scales. In particular we want them to connect what happens at the visible, human scale with what is going on at the atomic scale. We want students to see patterns in chemical processes based on the matter and energy changes associated with breaking and forming bonds. Perhaps most importantly, we want students to use the conservation laws as analytical tools to guide and constrain their explanations of chemical processes.
We do not know to what degree students’ thinking is con-text-specific. For example, we don’t know if students will think about all oxidation reactions in the same way that they think about combustion of organic fuels. However, we do have anecdotal evidence that similar issues apply when students attempt to connect abstract learning about chemical processes to macroscopic events. For example, teacher candidates who were science majors in a senior-level course explained that a pound of salt mixed with twenty pounds of water until all of the salt dissolved would weigh less than twenty-one pounds because the salt disappeared (Robinson and Nurrenbern, 2005). Other teacher candidates predicted that a rusty nail would weigh less than the original nail, because “rust is fuffy” (cf., Hesse & Anderson, 1992). Despite the fact that these teacher candidates proposed these explanations during a discussion where conservation of matter was the theme, they based their explanations on what they saw, setting aside conservation laws.
Implications for teachingWhat are the implications of the learning progression find-ings for teaching? First, very few students achieve Level 4 understanding of these processes. The biggest difference between Levels 3 and 4 is a commitment and ability to trace matter and energy. Tat is, Level 4 students use tracing matter and energy as analytical tools or crosscutting concepts (nrc, 2012) for examining processes. They are not satisfied with explanations that contradict the conservation laws, and they have the various pieces of knowledge needed to construct accurate accounts of matter cycling and energy flows. Level 3 students tend to have lots of pieces of knowledge but lack a sense of necessity in making their accounts conform to the conservation laws and/or they confound matter and energy. This implies that we should be focusing explicit instruction on helping students learn to use the conservation laws.
Rice, Doherty, & Anderson (in press) have shown that with non-science majors at the college level, when instruction explicitly and consistently uses tracing matter and energy as an organizational framework, more students advance to a Level 4 understanding than in classes that use less directed active learning (42% vs. 16%). In this class, students are repeatedly asked to account for matter and energy before and after a process. They use different colored paper clips to represent atoms and model molecules in reactants and products, going so far as to weigh them, demonstrating in a concrete way that they did not lose or gain mass during the process. They used strips of paper labeled with different types of energy to identify the associated energy transformations.
In the Carbon time curriculum, which is currently being developed in a partnership among Michigan State University, the National Geographic Society, and the Seattle Public Schools (http://edr1.educ.msu.edu/environmentallit/pub-licsite/html/CarbonTIME.html), explicit instruction about how to use the crosscutting concepts of matter and energy takes the form of rules (atoms last forever, atoms can be rearranged to form different molecules, energy lasts forever) and questions that students are routinely asked as they develop models for the processes they explore. The questions are: Where are atoms moving? What is happening to carbon atoms? What is happening to chemical energy? Students concretize the conservation laws by building and comparing models of the reactants and products. Twist ties around the C-C and C-H bonds signify chemical potential energy which is not associated with carbon dioxide.
In addition to explicit and consistent instruction on use of the conservation laws, careful and precise language by both teachers and students is another common factor of the instruction in these effective classrooms. For example, the difference between “the food was used to provide energy” and “the food was converted to energy” is subtle but important. Students often give subtle hints in their language about how they are thinking about chemical change. For example, “The match burned up” usually suggests Level 2 thinking. “The match burned” is an accurate statement that warrants a follow-up question to determine the student’s chemical understanding of “burned:” What happens to the atoms in the wood when the match burns?
Insistence on precise and consistent use of the conservation laws must extend beyond instruction and discussion to assessment. Not all assessment items will reveal students’ problematic thinking. In particular, students can learn to accurately answer questions that ask them to work at only one scale (macroscopic or microscopic/atomic) without needing to invoke an understanding of the conservation laws. For example, students may be able to balance the equation for combustion of octane (atomic molecular only) by using algorithms, yet answer questions about burning gasoline by saying that the gasoline is converted to energy. Even though we balance equations so that they conform to the law of conservation of matter, students may successfully balance the equation using a series of rules without understanding the reasoning. In contrast, the following question asks students to use knowledge of the combustion reaction to explain what happens to the molecules during the familiar process of burning gasoline: Gasoline is mostly a mixture of molecules such as octane: C8H18. Choose whether each of the following statements about what happens to the atoms in a molecule of octane when it burns inside a car is true (T) or false (F).T F
Some of the atoms in the octane become part of carbon dioxide in the air.
T F
Some of the atoms in the octane become part of air pollutants such as ozone (O3) or nitric oxide (NO2).
T F
Some of the atoms in the octane are converted into energy that moves the car.
T F
Some of the atoms in the octane are burned up and disappear.
T F
Some of the atoms in the octane are converted into heat.
T F
Some of the atoms in the octane become part of water vapor in the atmosphere.
Explain the pattern in your answers. What happens to the atoms in the octane when it burns inside a car?
The format of the questions is important here, too. The multiple true/false questions force students to deal with defini-tive statements that they often avoid when responding to essay questions. On the other hand, the final essay question reveals the nature of the students’ reasoning about how to use the conservation laws.
ConclusionIn summary, the research reported here on learning progressions for students’ use of the conservation laws to understand carbon-transforming processes explains why students’ accounts of basic processes differ in so many ways from scientific accounts. As students move from macroscopic accounts to accounts that examine events at the atomic level, they need to develop knowledge of the atoms in the substances being studied and the energy associated with the substances. In addition, they need to develop a sense of necessity around making their accounts adhere to conservation laws. This suggests that we can do a better job teaching if we explicitly use conservation laws as analysis tools and not just additional facts and we consistently use this approach in assessment as well as instruction.