Lymphadenectomy is recommended during surgery for papillary thyroid carcinoma when there is evidence of cervical lymph node metastasis (therapeutic) or in high-risk patients (prophylactic) such as those with T3 and T4 tumors of the TNM classification. Selective sentinel lymph node biopsy may improve preoperative diagnosis of nodal metastases.
ObjectiveTo analyze the results of selective sentinel lymph node biopsy in a group of patients with papillary thyroid carcinoma and no evidence of nodal involvement before surgery.
Patients and methodA retrospective, single-center study in patients with papillary thyroid carcinoma and no clinical evidence of lymph node involvement who underwent surgery between 2011 and 2013. The sentinel node was identified by scintigraphy. When the sentinel node was positive, the affected compartment was removed, and when sentinel node was negative, central lymph node dissection was performed.
ResultsForty-three patients, 34 females, with a mean age of 52.3 (±17) years, were enrolled. Forty-six (27%) of the 170 SNs resected from 24 (55.8%) patients were positive for metastasis. In addition, 94 (15.6%) out of the 612 lymph nodes removed in the lymphadenectomies were positive for metastases. Twelve of the 30 (40%) low risk patients (cT1N0 and cT2N0) changed their stage to pN1, whereas 12 of 13 (92%) high risk patients (cT3N0 and cT4N0) changed to pN1 stage.
ConclusionsSelective sentinel lymph node biopsy changes the stage of more than 50% of patients from cN0 to pN1. This confirms the need for lymph node resection in T3 and T4 tumors, but reveals the presence of lymph node metastases in 40% of T1–T2 tumors.
La linfadenectomía en la cirugía del carcinoma papilar de tiroides se aconseja cuando hay evidencia de metástasis ganglionar cervical (terapéutica) o en pacientes de alto riesgo (profiláctica), como en los tumores T3 y T4 de la clasificación TNM. La técnica de la biopsia selectiva del ganglio centinela puede mejorar el diagnóstico prequirúrgico de las metástasis ganglionares.
ObjetivoAnalizar el resultado de la biopsia selectiva del ganglio centinela en un grupo de pacientes con carcinoma papilar de tiroides T sin evidencia de afectación ganglionar antes de la cirugía.
Pacientes y métodoEstudio retrospectivo, unicéntrico en el que se incluyeron los pacientes intervenidos entre los años 2011-2013 que fueran clínicamente N0. La identificación del ganglio centinela se realizó mediante técnica isotópica. En todos los casos, se practicó linfadenectomía del compartimento afecto si el ganglio centinela era positivo, y del compartimento central en caso de ganglio centinela negativo.
ResultadosSe incluyeron 43 pacientes, 34 mujeres, con una edad media de 52,3 (±17) años. De los 170 ganglios centinela resecados, 46 (27%) fueron positivos para metástasis, que correspondían a 24 (55,8%) pacientes. En las linfadenectomías se resecaron 612 ganglios. De ellos, 96 (15,6%) fueron positivos para metástasis. Doce de los treinta (40%) pacientes cT1N0 y cT2N0 pasaron a pN1 tras la biopsia selectiva del ganglio centinela, mientras que 12 de los 13 (92%) pacientes cT3N0 y cT4N0, acabaron siendo pN1.
ConclusionesLa biopsia selectiva del ganglio centinela recalifica más del 50% de pacientes de cN0 a pN1. Se confirma la necesidad de vaciamiento ganglionar en los tumores T3 y T4, pero pone al descubierto la presencia de metástasis linfáticas en el 40% de los T1-T2.
Papillary thyroid carcinoma (PTC) is the most frequent endocrine tumor.1 This malignancy usually spreads via the lymphatic route; metastases in the regional lymph nodes are therefore common, particularly at central compartment level.2 It is currently a matter of debate whether preventive lymph node removal of the central compartment should accompany thyroidectomy as routine practice (prophylactic lymphadenectomy [PLA]), or whether lymph node removal should only be performed when the presence of lymph node metastases (LMs) has been confirmed either previously or during surgery (therapeutic lymphadenectomy [TLA]).3–5 Arguments in favor of PLA are the difficulty of diagnosing LMs before surgery; the fact that it avoids second surgery to perform lymphadenectomy; and the known relationship between LM and the risk of disease relapse. In contrast, purported drawbacks of PLA are the prolongation of surgery time; increased morbidity of thyroid surgery, particularly in relation to definitive hypoparathyroidism; and the fact that it does not modify survival.5 The current recommendations are to perform lymphadenectomy in all cases where the presence of LMs has been evidenced before or during surgery, and also in those cases where the risk of lymphatic spread is high—even if such spread has not been confirmed. In this respect, the American Thyroid Association6 guide, in its recommendation 36, advises PLA in stage T3 and T4 tumors according to the TNM classification of the American Joint Committee on Cancer.7
Since one of the key factors is to be able to plan intervention knowing the patient lymph node status, different strategies have been developed with the aim of improving the diagnosis of LMs before or during surgery. One such strategy is selective sentinel lymph node biopsy (SLNB).8,9 Detection of the sentinel lymph node (SLN) can be made using a radiotracer, a vital stain, the combination of both, or even magnetic particles. The first lymphatic drainage station is detected in all cases. Once identified, the SLN is removed during surgery and is immediately subjected to histological study in order to obtain a provisional verdict as to whether LM is present or not while surgery is still ongoing (intraoperative study). If LM is confirmed, lymphadenectomy of the corresponding compartment is performed.
The present study analyzes the results of SLNB in a group of patients with PTC without evidence of lymph node involvement (cN0) before surgery, and who were subjected to lymphadenectomy.
Patients and methodsPatientsA retrospective review was made of all the patients diagnosed with PTC between 2011 and 2013, and who met the following criteria: (1) no evidence of lymph node involvement before surgery (cN0 according to the TNM classification). In all cases this required the availability of a preoperative thyroid ultrasound study. (2) Patients subjected to SLNB; (3) effective SLNB, i.e., at least one SLN had to be identified; and (4) patients subjected to central compartment lymphadenectomy. In all cases in which SLNB proved positive, the affected compartment was subjected to lymphadenectomy, while if SLNB proved negative, lymphadenectomy of the central compartment homolateral to the tumor was performed as a strategy for validating the technique. All patients gave written informed consent to the aforementioned techniques.
Selective sentinel lymph node biopsyOn the day before the operation, 0.1–0.2ml of Tc99m-nanocolloid (Nanocoll®) was injected into the tumor under ultrasound guidance. The particle sizes were 50 and 80nm, and the radiotracer activity rating was 148MBq. After 2–4h SPECT/CT (General Electric, Infinia Hawkeye 4) was performed and the projection of the SLNs detected by the imaging test was marked on the skin. On the following day total thyroidectomy was performed, and the SLNs were subsequently located by means of a gamma radiation detector probe (Europrobe; Eurorad, Eckbolsheim, France). The lymph nodes were then resected and sent to the pathology laboratory. Lymphadenectomy of the central compartment homolateral to the tumor was then performed, along with lymphadenectomy of the lateral compartment in those cases with positive SLNs in the pathology study. The intraoperative analysis of the SLNs was made by cytological imprinting and the obtainment of frozen sections, in order to yield a provisional result. The definitive diagnosis of the SLNs was obtained on a delayed basis by analyzing histological sections according to levels of depth and stained with hematoxylin–eosin (HE). In addition, in those cases apparently without metastatic spread, a cytokeratin immunohistochemical study was made to discard the presence of small metastatic deposits not detected in the initial light microscopic evaluation of the HE-stained sections. Lymphadenectomy analysis was performed via the histological study of a central or core section of each lymph node stained with HE.
Statistical analysisQuantitative variables were reported as the median (interquartile range [IQR]), while categorical variables were reported as frequencies and percentages. The Mann–Whitney U-test was used for the comparison of medians. The relationship between quantitative variables was explored with the Pearson correlation statistic (r) and the coefficient of determination (R2). Statistical significance was considered for p≤0.05.
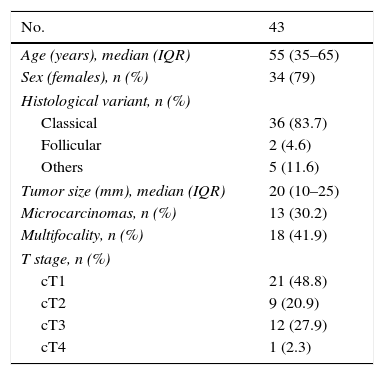
ResultsOf the 60 cases involved in validation of the SLNB technique in our center, 43 met the established study criteria. The main clinical–histological characteristics are summarized in Table 1.
Clinical–pathological characteristics of the patients.
| No. | 43 |
|---|---|
| Age (years), median (IQR) | 55 (35–65) |
| Sex (females), n (%) | 34 (79) |
| Histological variant, n (%) | |
| Classical | 36 (83.7) |
| Follicular | 2 (4.6) |
| Others | 5 (11.6) |
| Tumor size (mm), median (IQR) | 20 (10–25) |
| Microcarcinomas, n (%) | 13 (30.2) |
| Multifocality, n (%) | 18 (41.9) |
| T stage, n (%) | |
| cT1 | 21 (48.8) |
| cT2 | 9 (20.9) |
| cT3 | 12 (27.9) |
| cT4 | 1 (2.3) |
A total of 170 SLNs were resected, of which 139 corresponded to the central compartment (CC) and 31 to the lateral compartment (LC). The median number of SLNs per patient was 4 (IQR 2–5.4; minimum 1, maximum 9). Forty-six proved positive for metastasis (27%) – 40 in the CC and 6 in the LC – corresponding to 24 patients (55.8%), with a median number of positive nodes of 1.5 (1–3). There were no significant differences in the median number of resected SLNs between those cases finally found to be negative lymphadenectomies (3 [2–5]) and those found to be positive (4 [2–5]) (p=0.74).
A total of 43 and 7 lymphadenectomies of the CC and LC were performed, respectively. Twenty-one of the CC resections (48.8%) and 5 of the LC resections (71.4%) proved positive. Four patients showed disease involvement of both compartments, and a single patient showed involvement of the LC only. The lymphadenectomies yielded a total of 612 lymph nodes, with a median of 14 (8–18) nodes per patient. Of these lymphadenectomies, 96 were positive for metastasis (15.6%), with a median of 3 (1.5–5.5) positive nodes per lymphadenectomy. There were no differences in the number of lymph nodes per lymphadenectomy between those which proved positive (12 [7.2–18]) and those found to be positive (14.54 [11.5–17.5]) (p=0.42). A correlation was found between the number of SLNs and the number of nodes removed per lymphadenectomy (R2=0.46; p=0.002). In contrast, no correlation was found between the number of SLNs and the total positive nodes (R2=0.17; p=0.26), or between the number of positive SLNs and the number of removed lymph nodes (R2=0.23; p=0.13). Lastly, a correlation was observed between the number of positive SLNs and the number of removed positive nodes (R2=0.64; p<0.001).
Morbidity comprised two patients (4.6%) with temporary unilateral recurrent laryngeal nerve paralysis, four patients (9.3%) with transient hypoparathyroidism, and one patient with self-limiting cervical chyloma. There were no complications specifically associated to SLNB.
All patients were staged as cN0. Twelve of the 30 cT1 and cT2 cases (40%) were classified as stage pN1 after SLNB, while 12 of the 13 cT3 and cT4 cases (92%) were finally classified as stage pN1. In two cases the intraoperative study of the SLNs failed to detect metastasis. However, the delayed study of these nodes, as well as of the lymphadenectomies were seen to be positive in the final pathology report – thus yielding a false-negative rate of 8.3%. In contrast, there were no false-positive cases (i.e., a positive intraoperative study not confirmed by the definitive histological analysis). Lastly, in four patients (16.6%) the only positive node was the SLN—the rest of the lymphadenectomy proving negative.
DiscussionThe use of SLNB in PTC is subject to debate.10,11 The diversity of the protocols and of the patient characteristics; the different markers used to identify the SLN (dyes, radioisotopes, carbon nanoparticles or a combination of such markers)12,13; and the limitations of the intraoperative technique in detecting metastases,14,15 cause the results to differ greatly among the different published series. The isotopic technique is the option that affords the highest SLN localization percentages.12,16
The present study examines the effectiveness of the SLNB technique in application to the intraoperative staging of neck lymph node involvement in PTC. The objective of the technique is to identify those patients amenable to lymphadenectomy—the latter being of a therapeutic nature in all cases, since lymph node involvement would have been confirmed. Accordingly, we only selected those cases lacking any evidence of LM before surgery. Furthermore, as a procedure for initial validation of the technique, cervical central compartment lymph node removal was performed in all cases, independently of the SLNB result; the definitive histological report on lymph node status of the CC was therefore available. Although the number of patients was limited, the series was homogeneous (stage cN0); these therefore represented the prototype of patients with the dilemma of whether or not to perform PLA. Intraoperative analysis of the SLN demonstrated LM in 24 of the 43 patients (55.8%). This means that application of the technique resulted in a change in stage of disease (from cN0 to pN1) in over one-half of the cases. In a similar study involving 99 patients, Pelizzo et al.9 detected LM in 49% of the cases, while Chow et al.17 identified occult lymphatic metastases in 10 out of 15 patients (67%). Other authors have published lower percentages.15,18,19 As an example, in a small series of 23 subjects, Cabrera et al.18 reported a change in staging from cN0 to pN1 in 30.4% of the cases. This is similar to the observations of Larrad el al.8 and Ji et al.,15 who observed this change in 24 out of 114 patients (28.6%), though another 7 positive cases were identified in the definitive histological study of the SLN (6%). In a more recent study involving over 340 patients, Carcoforo et al.20 detected LM in 27% of the cases. The meta-analysis conducted by Balasubramanian and Harrison10 evidenced great differences among the included studies, with a mean SLN positivity rate of 43% in patients clinically corresponding to stage N0. One of the arguments in favor of PLA is the low sensitivity of the imaging techniques in the study of disease spread. Although ultrasound exploration of the neck offers high performance in the evaluation of thyroid nodules and even lateral neck adenopathies, it is much less effective in diagnosing lymph nodes of the CC (the most affected zone in PTC).21
According to the guide of the British Thyroid Association,22 prophylactic lymphadenectomy of the central compartment should be individualized in all high risk patients (defined as those meeting any of the following criteria: more aggressive histological variants; patients over 45 years of age; multifocal neoplasms; tumors measuring over 4cm in size or with spread beyond the thyroid gland). The recent guide of the American Thyroid Association6 in turn defines these patients as corresponding to stages T3 and T4 of the TNM classification. In our series, 30 of the 43 cases corresponded to groups cT1 and cT2. Of these, 12 (40%) changed to stage pN1. In contrast, 12 of the 13 cT3 and cT4 cases were also affected. These data confirm the high incidence of LM in the high risk stages—reinforcing the indication of PLA in patients with advanced disease. Nevertheless, the presence of lymph node involvement (40%) in the low risk patients raises doubts as to whether PLA should be performed in this group. In a study carried out by Maniakas et al.23 using SLNB with a dye-based localization technique, metastatic involvement was identified in only 11% of the T1-T2 cases versus in 58% of the T3–T4 cases.
Our study has important limitations. The few patients involved and the retrospective nature of the study require caution when interpreting the results. The patients with localized SLNs were included – these representing up to 95% of the cases in our experience.14 Although we did not have the definitive histological result, lymphadenectomy of the affected compartment was performed in cases with positive SLNs during the intraoperative study, or of the central compartment when the SLN proved negative. It is therefore not possible to determine the real involvement of the two compartments globally, since both were not subjected to node removal in all cases.
Since SLNB in PTC was first proposed by Kelemen et al.24 almost 20 years ago, its use has been the subject of intense debate. In this regard, the specific characteristics of this tumor and the high percentage of false-negative results have prevented SLNB from becoming a routine technique, in contrast to the situation found in other types of tumors.25–27 Despite the above, we conclude that SLNB affords more precise staging of lymph node involvement in patients with PTC. The presence of LM is frequent even in low risk patients. Nevertheless, the repercussions of this finding in relation to the long term prognosis are not clear.
Financial supportThis study was financed in part by a Research Grant for the Evaluation of Health Care Technologies and Services (PI09/90440) 2010–2012, principal investigator: Óscar González.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: González Ó, Zafon C, Caubet E, García-Burillo A, Serres X, Fort JM, et al. Biopsia selectiva del ganglio centinela en el carcinoma papilar de tiroides en pacientes sin evidencia preoperatoria de metástasis ganglionar. Endocrinol Diabetes Nutr. 2017;64:451–455.