Type 2 diabetes mellitus (DM2) has become a problem of global dimensions by their high and growing prevalence worldwide and the personal and economic costs associated with it. Correct treatment can reduce mortality and associated complications. New concepts have recently been included in routine clinical practice and have changed the algorithm of DM2 pharmacological therapy. Therefore, the Spanish Society of Diabetes (SED) entrusted to the Working Group of Consensus and Clinical Guidelines an update of the 2010 document Recommendations for Pharmacological Treatment of Hyperglycemia in Diabetes type 2. Novel aspects include nine characteristics to describe each drug group: efficiency, the risk of hypoglycemia, effects on body weight, the demonstrated effect in cardiovascular risk, nephroprotection, limitation of use in renal insufficiency, the rate of secondary effects, complexity and costs. Additionally, the document details combination options, and develop the start and adjustment of available injectable therapies.
La diabetes mellitus tipo 2 (DM2) es un problema de dimensiones globales por su alta y creciente prevalencia en todo el mundo y por los costes personales y económicos asociados a ella. Un tratamiento adecuado ha demostrado reducir la mortalidad y las complicaciones asociadas. Recientemente se han incluido nuevos conceptos en la práctica clínica habitual y en el árbol de decisión de la terapia farmacológica de la DM2. Por ello, la Sociedad Española de Diabetes (SED) encargó al Grupo de Trabajo de Consensos y Guías Clínicas actualizar el documento de 2010 «Recomendaciones para el tratamiento farmacológico de la hiperglucemia en la diabetes tipo2». Entre los aspectos novedosos se incluyen nueve características para describir a cada grupo farmacológico: eficacia, riesgo de hipoglucemia, efectos en el peso corporal, efecto demostrado en el riesgo cardiovascular, nefroprotección, limitación de uso en la insuficiencia renal, frecuencia de los efectos secundarios, complejidad y coste. Así mismo, se detallan las opciones de combinación y se desarrollan el inicio y el ajuste de las terapias inyectables disponibles.
Type 2 diabetes mellitus (DM2) is a major problem because of its high and increasing prevalence throughout the world and the personal and financial costs associated with the disease.1 Appropriate management, including lifestyle and pharmacological measures, has been shown to reduce DM2 related mortality2 and complications.3 The aim of reducing the adverse effects of the traditional therapeutic agents, as well as the need for different treatment options for such a complex and diverse disease, has greatly stimulated the development of new drugs.4
In 2010, the Steering Committee of the Spanish Society of Diabetes (Sociedad Española de Diabetes [SED]) commissioned the Consensus and Clinical Guidelines Working Group (Grupo de Trabajo de Consensos y Guías Clínicas [GTCGC]) to prepare a document embodying as far as possible the available evidence and the different recommendations regarding the situation of diabetes in Spain.5 The idea was to update this document regularly according to the emerging evidence and the suggestions of the members of the SED. Recently, the GTCGC was again commissioned to perform this task. Unlike the previous document, which was subject to a consensus with other scientific bodies with an interest in diabetes, the current document was prepared by the GTCGC of the SED. The SED is a scientific body which groups the different professionals involved in the management of DM2 and who are represented in the GTCGC, and consists of experts in endocrinology and nutrition, primary care, diabetes educators, pediatric endocrinology, and community pharmacy. We emphasize that these are recommendations made by the professionals of the working group based on an evaluation of the literature and their clinical experience, with the approval of the Steering Committee of the SED. However, the methodology used does not include stratification of the level of evidence and the strength of the recommendations.
In recent years there have been substantial changes in the available scientific evidence regarding agents for the treatment of DM2. Since 2008, the United States Food and Drug Administration (FDA) has required a full assessment of the cardiovascular safety profile of new antidiabetic treatments.6 During this period, many studies have been published involving new available drugs. The results have been heterogeneous and the methodology used has been the subject of debate.7,8 Furthermore, the incorporation of these results into the international clinical guides has been disparate. Some guides explicitly recommend assigning priority to drugs with favorable results in cardiovascular safety studies,9 though most of them reduce the applicability of this recommendation to patients with DM2 and established cardiovascular disease.9,10 We think that the available results afford knowledge that may be used in management algorithms, and that the drugs supported by them should be prioritized.
The present document thus includes the new concepts in standard clinical practice and in the drug therapy decision tree in DM2, such as a reduction of the aforementioned cardiovascular morbidity and mortality, the intrinsic complexity of the new agents, and the associated costs. However, the constant appearance of new pharmacological agents and the scientific evidence makes it advisable that these recommendations be updated on a regular basis. The aim is to do so each year.
Lastly, it should be noted that the recommendations, guidelines and administrative limitations of the local authorities may differ from one Spanish Autonomous Community to another, and from those included in this document.
Control targetsAchieving good glycemic control may avoid or delay the appearance of micro- and macrovascular complications, as has been shown by different studies involving long periods of follow-up.11 It is therefore recommended that very strict control should be achieved in the early stages of diabetes management (glycosylated hemoglobin [HbA1c] <6.5% as the optimal target). Under real life conditions this means achieving fasting glycemia levels of <125mg/dl and postprandial levels of <145mg/dl.12 However, different adverse effects, and in particular the risk of hypoglycemia, may increase when more intensive control is sought. Both susceptibility to and the severity of the consequences of these adverse effects are greater in people with:
- •
Important comorbidity, especially of a cardiovascular nature.
- •
Older age.
- •
A long duration of DM2.
- •
Irregular food intake.
- •
Inadvertent hypoglycemia.
- •
Renal failure (RF).
- •
Poor adherence to therapy.
- •
Personal limitations regarding the correct following of treatment.
- •
Frailty.
It is therefore advisable to make the control targets in these cases flexible and to consider an HbA1c objective or target of <8%, a basal glycemia of <140mg/dl and a postprandial glycemia of <200mg/dl.13 These targets do not mean that we should not seek the best glycemic control possible by means of antidiabetic therapy lacking hypoglycemia risk and scantly aggressive for the patient. Clinical judgment is irreplaceable for the individualization of these targets.
Therapeutic inertiaDelayed decision-making in the adjustment of DM2, so-called “therapeutic inertia”, is one of the main causes of failure to reach the established targets.14 After the start of treatment, or after successive treatment adjustments, we should re-evaluate the clinical condition of the patient, the new pharmacological treatment options available, and the glycemic control achieved. It is important for this process to be continuous and frequent, in view of the variability of response and the progressive nature of DM2.
The new drug classes may have frequent and especially early-onset adverse effects, and may require concomitant therapy adjustments. We therefore recommend the checking of tolerability and efficacy (through the self-monitoring of blood glucose [SMBG]) in the first four weeks following the introduction of a new drug and again after three months in order to confirm its suitability, based on glycemic (HbA1c and SMBG) and the clinical control parameters. Subsequently, once the targets have been achieved, all patients should be monitored at least twice a year. Therapeutic education is a key element in this continuous process, particularly when new and more complex drugs are introduced, such as those administered through injection. Active patient involvement in the management of the new treatment is crucial. This is especially true for injectable therapies and, in the case of insulin, patient self-adjustment of the dose has been shown to be both safe and effective.11,15 Diabetes education programs in such cases should include the changes in treatment required in the event of acute intercurrent conditions that may cause a degree of dehydration or produce eating problems.16
Results of clinical trials with cardiovascular outcomesAlthough it has been known for decades that improved glycemic control reduces the incidence of microvascular complications,11 scientific evidence regarding macrovascular complications and mortality has been lacking until very recently. There have even been concerns about the cardiovascular safety of some antidiabetic treatments.17 In addition, the results of trials comparing therapy seeking strict control (HbA1c<6.5%) versus conventional treatment in patients with DM2 and a high cardiovascular risk have questioned the safety of the former approach and of glucose-lowering therapy in general in DM2.18
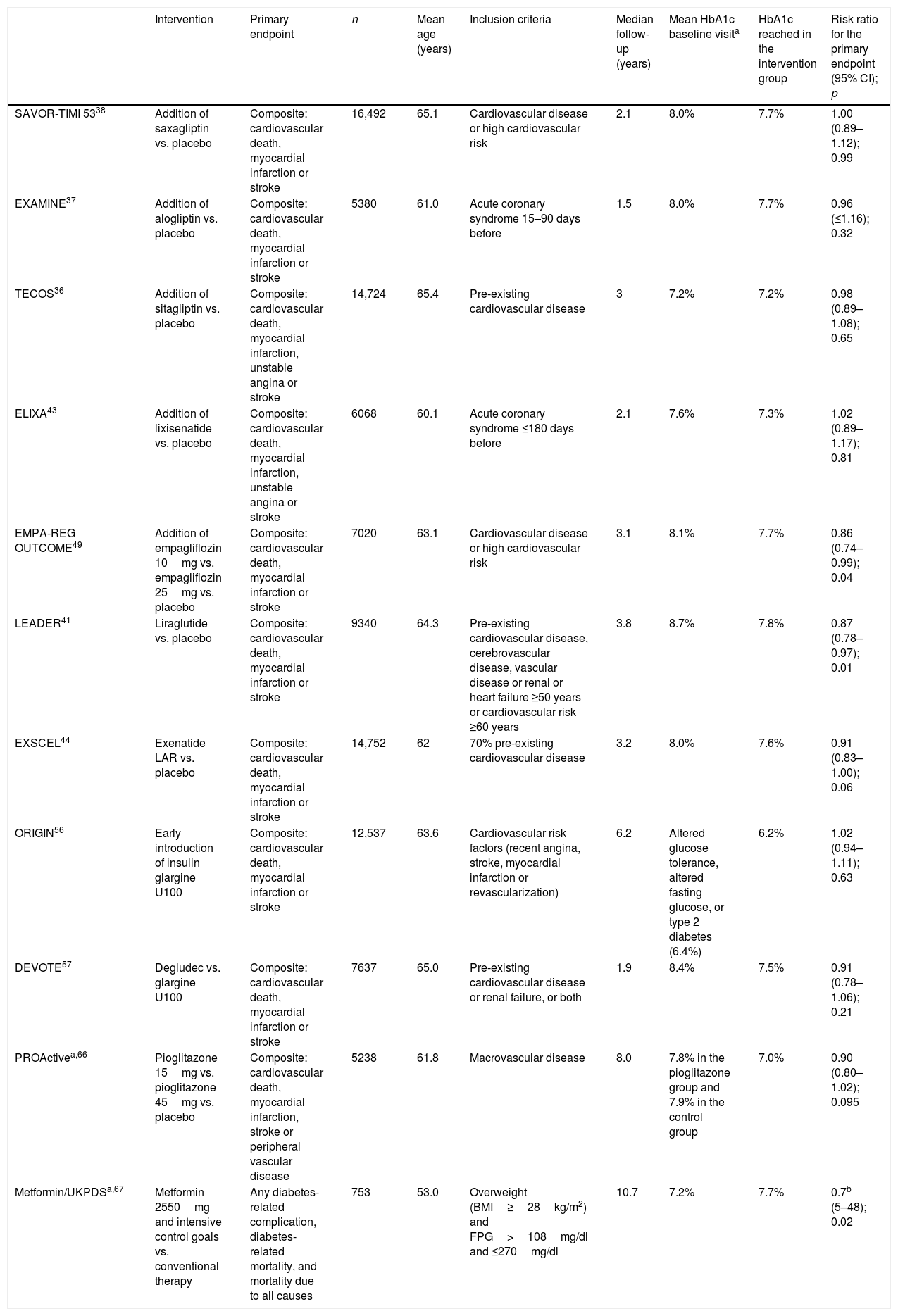
Since 2008, the FDA has required a full assessment of the cardiovascular safety profile of new antidiabetic treatments.6 During this period, a number of cardiovascular outcome trials (CVOTs) have been published involving the new drugs (Table 1). Although we would prefer to have more extensive information allowing for an increased external validity of cardiovascular safety studies, as well as the confirmation of causality through studies specifically designed for this purpose, we consider that the existing results offer useful information. Some of the most recent decision trees have incorporated the criterion of reducing the risk of cardiovascular morbidity and mortality with those drug substances that have been shown to be effective, particularly in the studied population groups (patients with established cardiovascular disease or at a very high cardiovascular risk).
Key aspects of the cardiovascular outcome trials with antidiabetic drugs in patients with DM2.
| Intervention | Primary endpoint | n | Mean age (years) | Inclusion criteria | Median follow-up (years) | Mean HbA1c baseline visita | HbA1c reached in the intervention group | Risk ratio for the primary endpoint (95% CI); p | |
|---|---|---|---|---|---|---|---|---|---|
| SAVOR-TIMI 5338 | Addition of saxagliptin vs. placebo | Composite: cardiovascular death, myocardial infarction or stroke | 16,492 | 65.1 | Cardiovascular disease or high cardiovascular risk | 2.1 | 8.0% | 7.7% | 1.00 (0.89–1.12); 0.99 |
| EXAMINE37 | Addition of alogliptin vs. placebo | Composite: cardiovascular death, myocardial infarction or stroke | 5380 | 61.0 | Acute coronary syndrome 15–90 days before | 1.5 | 8.0% | 7.7% | 0.96 (≤1.16); 0.32 |
| TECOS36 | Addition of sitagliptin vs. placebo | Composite: cardiovascular death, myocardial infarction, unstable angina or stroke | 14,724 | 65.4 | Pre-existing cardiovascular disease | 3 | 7.2% | 7.2% | 0.98 (0.89–1.08); 0.65 |
| ELIXA43 | Addition of lixisenatide vs. placebo | Composite: cardiovascular death, myocardial infarction, unstable angina or stroke | 6068 | 60.1 | Acute coronary syndrome ≤180 days before | 2.1 | 7.6% | 7.3% | 1.02 (0.89–1.17); 0.81 |
| EMPA-REG OUTCOME49 | Addition of empagliflozin 10mg vs. empagliflozin 25mg vs. placebo | Composite: cardiovascular death, myocardial infarction or stroke | 7020 | 63.1 | Cardiovascular disease or high cardiovascular risk | 3.1 | 8.1% | 7.7% | 0.86 (0.74–0.99); 0.04 |
| LEADER41 | Liraglutide vs. placebo | Composite: cardiovascular death, myocardial infarction or stroke | 9340 | 64.3 | Pre-existing cardiovascular disease, cerebrovascular disease, vascular disease or renal or heart failure ≥50 years or cardiovascular risk ≥60 years | 3.8 | 8.7% | 7.8% | 0.87 (0.78–0.97); 0.01 |
| EXSCEL44 | Exenatide LAR vs. placebo | Composite: cardiovascular death, myocardial infarction or stroke | 14,752 | 62 | 70% pre-existing cardiovascular disease | 3.2 | 8.0% | 7.6% | 0.91 (0.83–1.00); 0.06 |
| ORIGIN56 | Early introduction of insulin glargine U100 | Composite: cardiovascular death, myocardial infarction or stroke | 12,537 | 63.6 | Cardiovascular risk factors (recent angina, stroke, myocardial infarction or revascularization) | 6.2 | Altered glucose tolerance, altered fasting glucose, or type 2 diabetes (6.4%) | 6.2% | 1.02 (0.94–1.11); 0.63 |
| DEVOTE57 | Degludec vs. glargine U100 | Composite: cardiovascular death, myocardial infarction or stroke | 7637 | 65.0 | Pre-existing cardiovascular disease or renal failure, or both | 1.9 | 8.4% | 7.5% | 0.91 (0.78–1.06); 0.21 |
| PROActivea,66 | Pioglitazone 15mg vs. pioglitazone 45mg vs. placebo | Composite: cardiovascular death, myocardial infarction, stroke or peripheral vascular disease | 5238 | 61.8 | Macrovascular disease | 8.0 | 7.8% in the pioglitazone group and 7.9% in the control group | 7.0% | 0.90 (0.80–1.02); 0.095 |
| Metformin/UKPDSa,67 | Metformin 2550mg and intensive control goals vs. conventional therapy | Any diabetes-related complication, diabetes-related mortality, and mortality due to all causes | 753 | 53.0 | Overweight (BMI≥28kg/m2) and FPG>108mg/dl and ≤270mg/dl | 10.7 | 7.2% | 7.7% | 0.7b (5–48); 0.02 |
FPG: fasting plasma glucose.
The drug treatment options for DM2 have increased considerably in recent years, allowing for a greater individualization of therapy. The new drugs offer undeniable advantages (particularly a lesser risk of hypoglycemia and weight gain) and explore new mechanisms of action not previously contemplated in therapy. By contrast, their effectiveness is conditioned by kidney function or neuroregulatory responses that are difficult to predict. Some of their benefits or adverse effects have been attributed to the global drug class or group involved (the so-called class effect), while others have only been seen in some concrete drug substances belonging to a given class. Moreover, the beneficial effects in terms of lowered blood pressure, intravascular volume or blood glucose also make it mandatory to review the concomitant therapy of the patient at the start of these antidiabetic treatments. Diuretics, antihypertensive agents and, especially, insulin may require changes that are not always easy to make. Combination therapy, which in the past was limited to two agents, has now been multiplied up to three or four times.
All the above has clearly increased the complexity of pharmacological treatment. This is something that cannot be avoided, and which the prescribing professionals (many of them not endocrinologists) see as causing difficulties for the intensification of therapy. While the present guide, like other international guides, is intended to facilitate these decisions, it is not always easy for professionals to adequately assimilate these new developments. Nevertheless, complexity should not be viewed as a barrier against the use of new treatments in those patients who may benefit from them. Effective interaction among all members of the interdisciplinary team should serve to improve access to these new drugs and combination therapies.
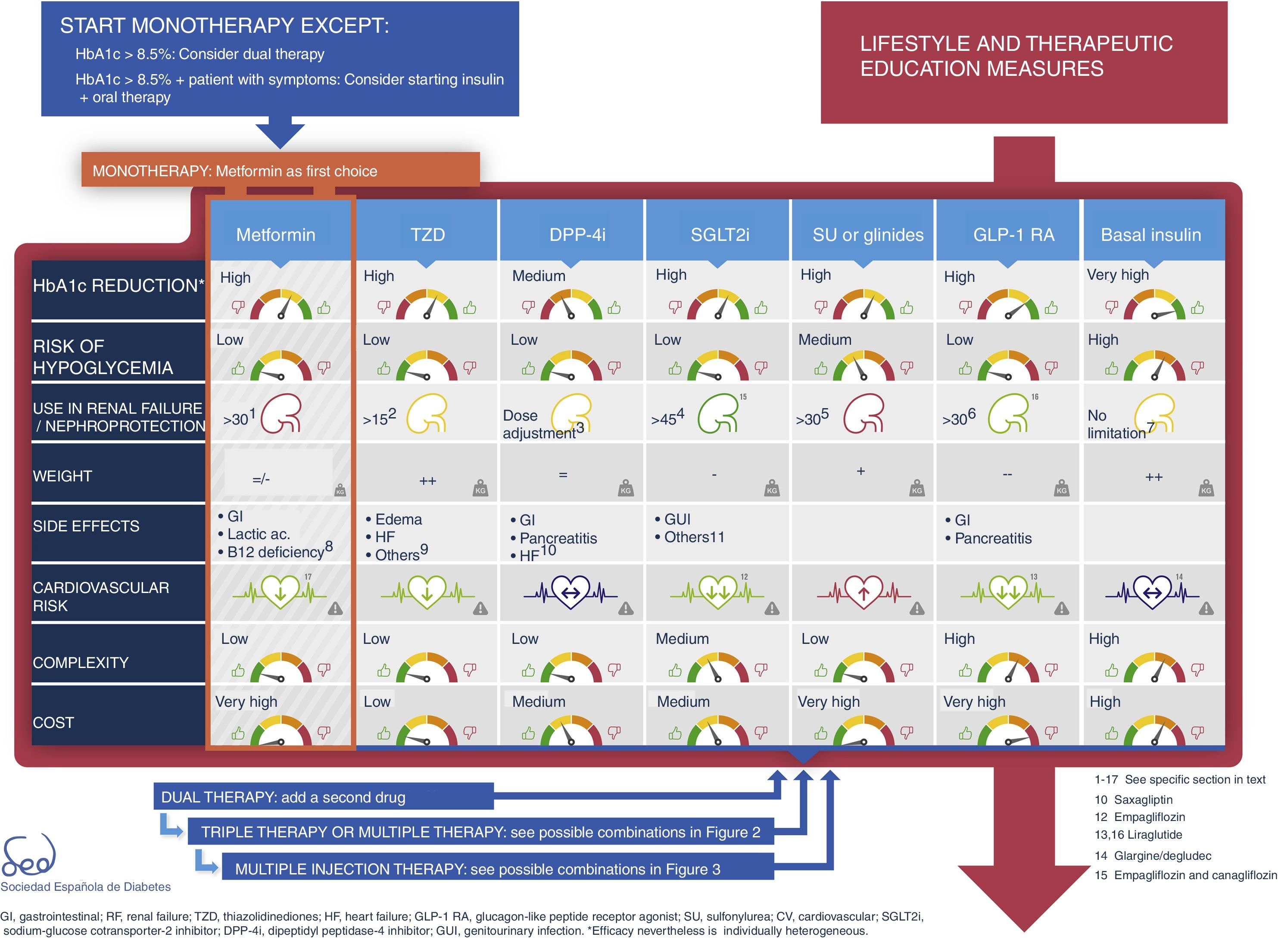
First treatment and early combinationIn some cases the control target (HbA1c<6.5%) can be achieved with changes in lifestyle alone,19 though this strategy is not always effective and depends on the characteristics of the patient and his or her adherence to the recommendations made. For this reason, the SED recommends the concomitant use of metformin from the start for most patients.5
In any case, the introduction of metformin should not be delayed for longer than three months if the individualized control target has not been reached.
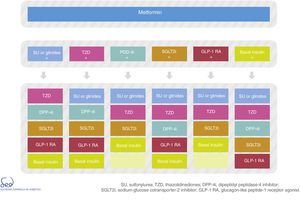
The physiopathology of DM2 includes alterations in a number of mechanisms and organs.20 This results in the almost universal need and desirability for combination treatment throughout the natural course of the disease. However, the use of two different drugs simultaneously may make it difficult to identify which is the cause of side effects. Thus, there are differences in criterion as to when combination drug treatment should be started after diagnosis, based on consensus-based HbA1c cut-off points. This update proposes two situations in which initial combination drug therapy may be considered (Fig. 1):
- •
HbA1c<8.5%.
- •
HbA1c>8.5% in symptomatic patients. In this case, we recommend that, together with oral therapy, insulin therapy should be considered. Once control and the clinical condition have improved, the insulin therapy may be discontinued.
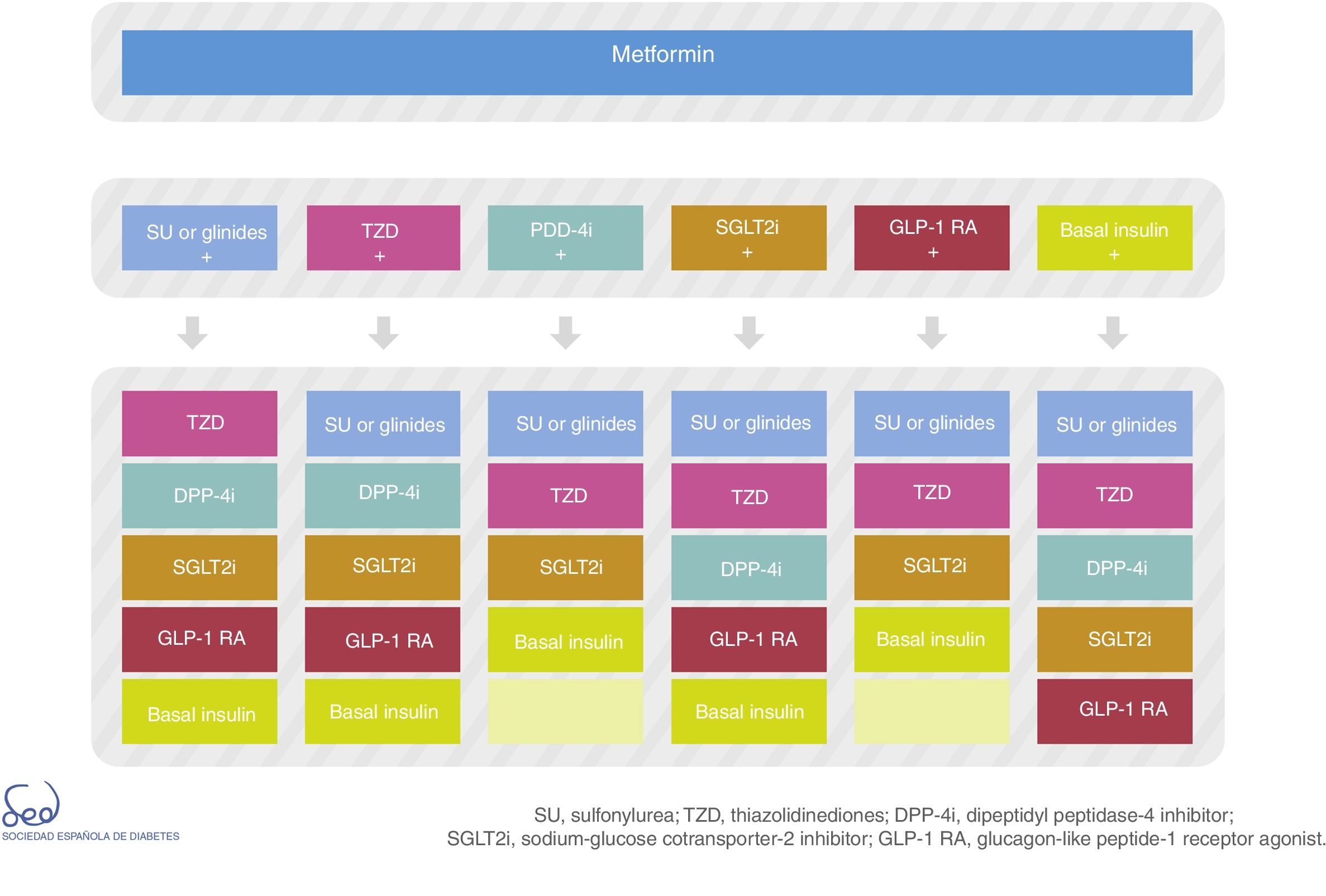
There are currently a number of combination treatment options (Fig. 2); some are available in oral tablet form, and combinations of drugs for injection may even be used. Clinical judgment and patient characteristics and preferences are essential factors to be taken into account when the most appropriate treatment option is being selected.
Description of drug classesMetforminMetformin is an effective drug, affording an expected mean HbA1c reduction of 1.3% to 2.0%, particularly when routinely used as the first option at the diagnosis of DM2 with elevated HbA1c levels.21 Despite extensive experience in the use of the drug, we have only recently advanced in our knowledge of its mechanism of action. Metformin, by binding to specific receptors, activates AMP kinase; liver glucose production decreases22 and intestinal glucose uptake increases, acting as a hyperglycemia clearance mechanism in diabetes.23 The effect of metformin on body weight is neutral, though in some cases it induces modest weight loss.24 While it is a safe drug, its gastrointestinal side effects appear to be underestimated in the literature.4 Gradual dose titration and administration with food is advised in order to improve tolerance of this drug. If intolerance is observed, the dose should be lowered again to the previous tolerated dose, followed by a subsequent increase. Although the capacity of metformin to lower cardiovascular risk (CVR) is accepted as proven, the available evidence is limited.25 In relation to renal failure, when the estimated glomerular filtration rate (eGFR) is <45ml/min, starting metformin is not advised, and if the drug has already been introduced, its dose should be lowered. Metformin should be discontinued if eGFR drops to below 30ml/min.26 The use of this drug is associated with vitamin B12 deficiency. Consequently, it has been advised that vitamin B12 levels and/or the presence of anemia or peripheral neuropathy should be regularly evaluated.27 Metformin is a low cost and low complexity drug.
Sulfonylureas and glinidesThis drug class has been used for decades. Although it is effective (a mean expected HbA1c reduction of 0.79% when added to metformin),28 the middle-term sustainability of treatment has been questioned.29 These drugs close the K-ATP channels in the beta-cell membrane, stimulating insulin secretion. This mechanism is independent of the plasma glucose levels, which conditions its associated hypoglycemia risk (considered to be average in view of the low frequency of hypoglycemia, but with occasional severe cases). A systematic review and meta-analysis showed a mean weight gain of 2.31kg in the context of sulfonylurea therapy.30 Glibenclamide is to be avoided in situations of renal failure, and lower doses should be used in the case of second-generation sulfonylureas (gliclazide, glimepiride). In patients with renal failure it is advisable not to start treatment in the presence of eGFR<45ml/min, and treatment should be discontinued in the case of eGFR<30.26 The metabolites of repaglinide are excreted mainly in bile; consequently there are no limitations on its use in patients with renal failure. These substances are also characterized by an early bioavailability peak and a shorter half-life. Consequently, they can offer better postprandial glycemic control than the sulfonylureas.31
Due to their risk of hypoglycemia and weight gain, and a potential increase in cardiovascular mortality,32 these drug substances are not a preferred option. However, because of their low complexity of use and cost-effectiveness, these agents are still considered to be adequate in patients at a low risk of hypoglycemia.33
Alpha-glucosidase inhibitorsThese drugs inhibit the intestinal alpha-glucosidases, reducing carbohydrate digestion and absorption. In some cases the alpha-glucosidase inhibitors may induce a slight weight loss, but they do not cause hypoglycemia when administered in monotherapy. However, these drugs are currently little used, because they are less potent than the other available options (mean expected HbA1c reduction of 0.65% when added to metformin),28 and cause frequent gastrointestinal problems (mainly flatulence), leading to treatment discontinuation in a high proportion of patients.34
Pioglitazone (thiazolidinediones)The thiazolidinediones act by activating the nuclear transcription factor PPAR-gamma, thereby increasing insulin sensitivity. When added to metformin, an HbA1c decrease of 1% can be expected.28 No dose adjustments are required in patients with renal failure, and the treatment can be used in the presence of eGFR>15ml/min/1.73m2. Their use has been limited by the presence of adverse effects such as weight gain, water retention (edema, heart failure), bone fractures and a purported association with bladder cancer.24 Consequently, these drugs are usually reserved for use in the third therapeutic step. Their favorable effect upon steatosis and non-alcoholic steatohepatitis is regarded as an advantage, however35, and they have been proposed as an alternative in cases of metformin intolerance/contraindication. They represent a low cost and low complexity treatment.
Dipeptidyl peptidase-4 (DPP-4) inhibitorsThese drugs inhibit DPP-4 enzyme activity, increasing the endogenous levels of incretin hormones (glucagon-like peptide-1 [GLP-1], gastric inhibitory polypeptide [GIP]) after food intake. Thanks to this mechanism of action, the DPP-4 inhibitors can increase insulin secretion and decrease glucagon secretion in a glucose-dependent manner. When added to metformin, the expected mean decrease in HbA1c is 0.79%.28 These drugs do not cause hypoglycemia when administered in monotherapy. The effect of the DPP-4 inhibitors on body weight is neutral, though in some cases they induce a slight weight loss. They can be safely used in any stage of chronic renal failure. Except for linagliptin, which is eliminated via the biliary route, all other DPP-4 inhibitors require dose adjustments in cases of moderate or severe renal failure, though this recommendation is made for pharmacokinetic reasons, not for reasons of safety. The DPP-4 inhibitors sitagliptin, alogliptin and saxagliptin have demonstrated cardiovascular safety in clinical trials involving patients at a high cardiovascular risk.36–38
In the SAVOR-TIMI 5 study, saxagliptin was associated with a significant increase in hospital admission due to heart failure,38 a situation not observed with the other molecules. Their cost is high, but lower than that of the recently marketed treatments for injection. The safety and the convenience of use (once-daily or twice-daily doses in combination with metformin) of the DPP-4 inhibitors have made them one of the most commonly used drug options, particularly in the early stages of the disease, in patients with renal failure, and in elderly subjects.39
Glucagon-like peptide-1 (GLP-1) receptor agonistsThese drugs bind to the GLP-1 receptors, inducing a decrease in glucagon secretion and an increase in insulin secretion, both in a glycemia dependent manner. They also slow gastric emptying and increase satiety. When added to metformin, the GLP-1 receptor agonists achieve a mean HbA1c reduction of 0.99%,28 together with a significant weight loss (expected −2.9kg on average).40 The GLP-1 receptor agonists do not induce hypoglycemia when administered in monotherapy. They lower both systolic (−3.57mmHg) and diastolic blood pressure (−1.38mmHg).40
In general, the GLP-1 receptor agonists are not recommended in the presence of eGFR<30ml/min, with the exception of liraglutide and dulaglutide, which can be used in situations as low as eGFR 15ml/min. The experience gained in cases of renal failure is more limited with the remaining drugs, though no dose adjustments are required. The LEADER trial showed liraglutide to reduce a composite renal endpoint (defined as new-onset macroalbuminuria or a doubling of serum creatinine, and an eGFR of ≤45ml/min/1.73m2, the need for renal replacement therapy, or death due to renal failure).41 However, most of this benefit occurred through the first of the mentioned parameters, i.e., macroalbuminuria, which is a surrogate marker of kidney disease. Thus, the potential arrest of renal disease progression (nephroprotection) with liraglutide is more uncertain than with empagliflozin/canagliflozin (see below), and studies with longer follow-up periods than the LEADER trial are needed to reveal differences in the “hard” treatment targets.42 Furthermore, liraglutide has been shown to reduce the main cardiovascular adverse events (stroke and acute myocardial infarction) and cardiovascular mortality in patients at a high cardiovascular risk,41 while lixisenatide43 and exenatide administered on a weekly basis have shown cardiovascular neutrality.44
Approximately 20% of all patients experience gastrointestinal side effects (mainly nausea and, to a lesser extent, vomiting) at the start of therapy, though this incidence decreases in the following weeks, and treatment discontinuation is required in only a few cases (3–8%).45 The use of GLP-1 receptor agonists has been associated with a slightly increased risk of acute pancreatitis, though such events have been infrequent, and statistical significance has not been reached in the clinical trials. Patients should be informed of the characteristic symptoms of acute pancreatitis and, if the latter is suspected, treatment should be discontinued. These drugs are expensive, and their reimbursement in the public healthcare system is limited to people with a body mass index (BMI) of >30kg/m2. Subcutaneous administration is required (daily in two doses for exenatide, one for liraglutide and lixisenatide, and weekly for exenatide-LAR [long-acting release] and dulaglutide), as well as training regarding the potential adverse effects. These are therefore considered to be high complexity drugs.
Sodium-glucose cotransporter 2 (SGLT2) inhibitorsThese drugs prevent glucose reabsorption in the convoluted proximal renal tubule by blocking the sodium-glucose cotransporter 2 (SGLT2), thus inducing glucosuria. The expected decrease in HbA1c is 0.7–1%,46 and sustained efficacy has been demonstrated in studies with up to fouryears of follow-up.47 Since their mechanism of action is insulin-independent, the SGLT2 inhibitors are effective in all stages of DM2 and do not cause hypoglycemia when administered in monotherapy. These drugs induce a mean weight loss of −1.88kg and also lower blood pressure (mean: −2.46mmHg in systolic and −1.46mmHg in diastolic blood pressure).48 The EMPA-REG,49 CANVAS and CANVAS-R trials,50 conducted in patients with DM2 and high cardiovascular risk, showed empagliflozin and canagliflozin to reduce cardiovascular risk (including cardiovascular death, non-fatal myocardial infarction or non-fatal stroke, and hospital admission due to heart failure). In the CANVAS study, treatment with canagliflozin was associated with an increased risk of amputations (primarily of the toe or metatarsals) and fractures.50 Fractures were more common in the limbs. However, on excluding fractures of the hand, foot, skull and face (i.e., sites not associated with osteoporosis or skeletal fragility), the incidence of fractures with canagliflozin no longer reached statistical significance.51
Efficacy decreases with eGFR<60ml/min/1.72m2. Dapagliflozin should not be administered below this filtration limit, while empagliflozin and canagliflozin can be used with eGFR between 45 and 60ml/min/1.72m2, provided the dose is lowered. Administration should be discontinued in the presence of eGFR<45ml/min/1.72m2.
Empagliflozin resulted in reductions in incident or worsening nephropathy, a doubling of serum creatinine levels and renal replacement therapy in a pre-specified analysis of the EMPA-REG trial.52 Likewise, the results of the CANVAS study showed a possible benefit of canagliflozin in terms of the progression of albuminuria and a composite endpoint that included a sustained 40% reduction in GFR, the need for renal replacement therapy, or death due to renal causes.50 In contrast to the results previously reported with liraglutide, those afforded by the SGLT2 inhibitors included robust targets. This, combined with the renal hemodynamic actions of this drug class, makes an intrinsic nephroprotective effect likely.42
The most common adverse effects of these drugs are genitourinary infections, particularly in women. Glucosuria causes osmotic diuresis and polyuria; adverse events due to volume depletion have therefore been observed, particularly in the elderly and with the concomitant use of diuretics. Hypoglycemia may occur when SGLT2 inhibitors are combined with sulfonylureas or insulin. All these adverse effects may manifest at the start of treatment. It is therefore advisable to proactively adjust glucose-lowering and antihypertensive treatment.53 Rare cases of euglycemic ketoacidosis have been reported, especially in patients receiving insulin.54
Sodium-glucose cotransporter 2 inhibitors are administered as a once-daily oral dose (or twice-daily in combination with metformin). However, the need to adjust concomitant therapies and the frequency of adverse events defines SGLT2 inhibitors as medium-complexity therapy.
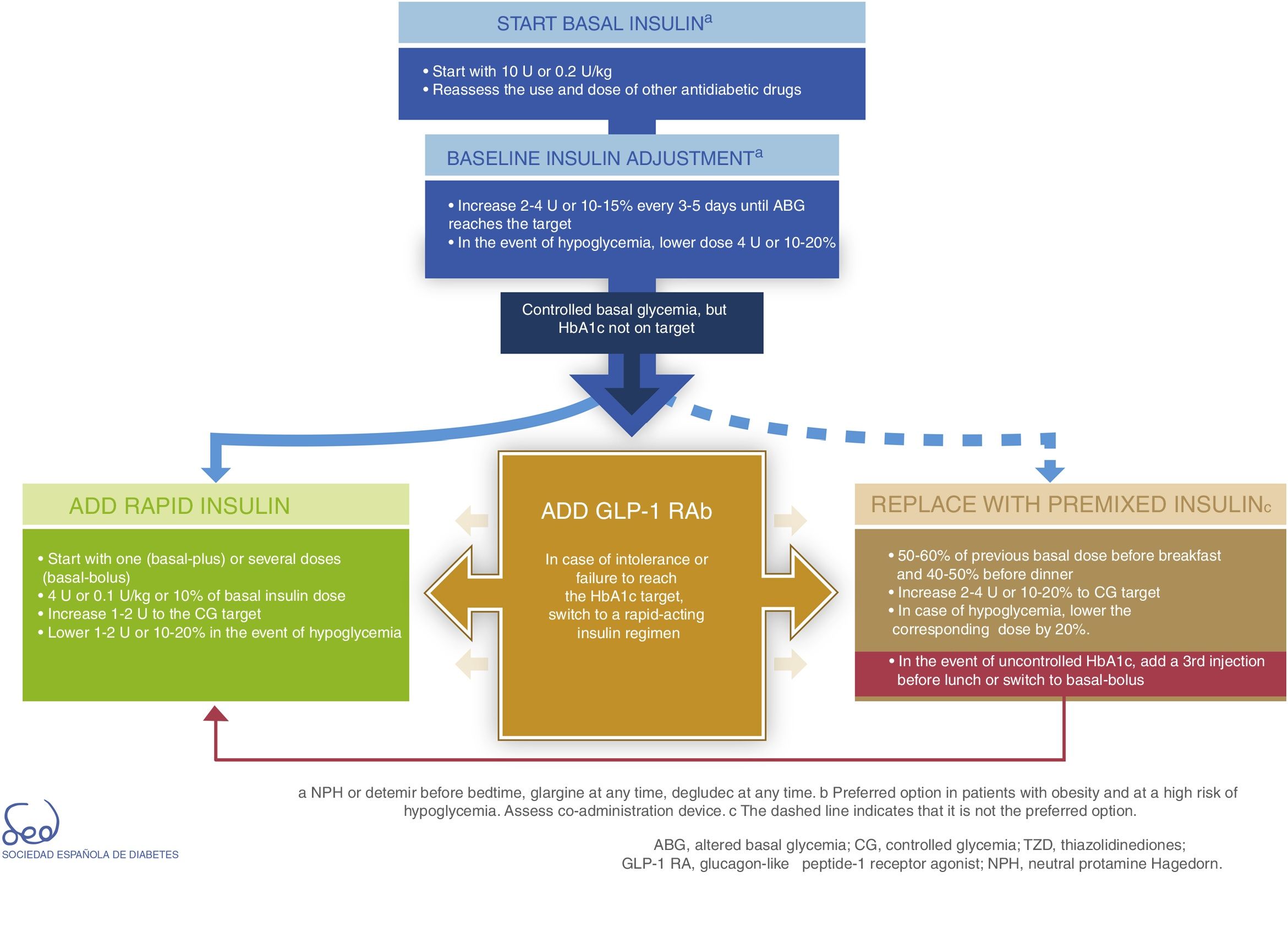
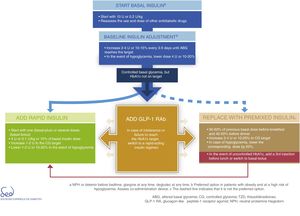
InsulinDirect administration of the hormone that is deficient in DM2 (insulin) is the most potent glucose-lowering option, particularly in insulinopenic patients, in whom the resulting expected mean HbA1c reduction is between 1 and 2%. Insulin therapy is also associated with an increased risk of hypoglycemia. It acts by decreasing glucose production in the liver and favoring peripheral glucose utilization. The proposal for therapeutic progression from non-insulin drugs to insulin therapies and their intensification is described in Fig. 3.
Weight gain with insulin is greater than with other current therapeutic options. In observational studies, insulin use was associated with a weight gain of up to 6kg in two years.55
Insulin is not contraindicated in patients with renal failure, though its dose should be lowered in such cases. Cardiovascular safety was demonstrated in the ORIGIN (glargine)56 and DEVOTE (degludec) trials.57
Basal insulin analogs (glargine U100 and U300, detemir, degludec) have shown a lesser risk of hypoglycemia (particularly nocturnal hypoglycemia) than human NPH insulin.58 The cost of such treatment is higher, and insulin degludec is the most expensive, its prescription within the public healthcare system currently being limited to authorized cases only. Degludec and glargine U300 have shown a lesser incidence of hypoglycemia (particularly nocturnal hypoglycemia) compared with insulin glargine U100, as well as a greater flexibility in the timing of doses.59,60
Basal insulin is regarded as the first insulin therapy option. It is advisable to continue treatment with metformin in order to reduce the insulin requirements. While the decision to continue other oral antidiabetic drugs or GLP-1 receptor agonists should be made on an individualized basis, they may afford better postprandial control and reduce the insulin requirements, which in turn is associated with a lesser weight gain. The “Consensus on insulin treatment in type 2 diabetes”, recently published by this same GTCGC, recommends maintaining DPP-4 inhibitors and SGLT2 inhibitors, and reducing/discontinuing sulfonylureas and pioglitazone.61 When starting basal insulin, the recommended daily dose is 0.2U/kg or 10U. Dose adjustments should be made gradually, with increments of 2–4U or 10–15% every 3–5 days until the fasting glycemic target has been reached. If unexplained hypoglycemia occurs, the dose should be lowered 4U or 10–20%.61
When the glycemic target has been reached but not so the HbA1c target, an intensification of the therapy is recommended. The combination of basal insulin with GLP-1 receptor agonists affords similar glycemic control with a lesser risk of hypoglycemia as compared to other insulin regimens, and is therefore currently considered a preferential option (Fig. 3).62
In case of intolerance, if the HbA1c target is not reached, or if directly not considered a valid option, we can choose a rapid-acting insulin regimen. In these cases, the most advisable strategy is to use rapid insulin boluses. Treatment can be started as a single dose with the most important hyperglycemic excursion (basal-plus) or directly in several doses (basal-bolus). The dose recommendation for these boluses is 4U or 0.1U/kg or 10% of the basal insulin dose. Dose titration is made through the postprandial self-monitoring of blood glucose (SMBG), incrementing 1–2U to target. Dose reduction by 1–2U or 10–20% is indicated if unexplained hypoglycemia occurs.61
The premixed insulin option is associated with an increased risk of hypoglycemia and weight gain, and requires regular timings and intake and physical activity patterns.63 When starting premix insulins, 50–60% of the previous basal insulin dose can be used before breakfast and 40–50% before dinner. For titration, it is advisable to increase 2–4U or 10–20% to target glycemia. In the event of hypoglycemia, the corresponding dose should be lowered by 20%. If HbA1c is not controlled, a third injection may be added before lunch. If adequate control is not achieved, a switch to a basal-bolus strategy can be made.
All insulin treatment options are considered to be complex because of the need for subcutaneous injection and dose titration, the risks involved, and the need for specific diabetes education.
Fixed-dose drug combinationsIn recent years, combination options involving two drugs in a single tablet have been incorporated into DM2 therapy in order to simplify the treatment regimen and thus improve patient adherence. Oral combinations of metformin with sulfonylureas, pioglitazone, DPP-4 inhibitors or SGLT2 inhibitors are currently available, though there are also combinations of DPP-4 inhibitors with SGLT2 inhibitors, or pioglitazone with DPP-4 inhibitors. Experience indicates that they are well accepted due to their convenience and safety. These combinations have also been able to reduce therapeutic inertia, facilitating faster access to combination therapy.
The European Medicines Agency (EMA) has recently approved the following fixed combinations of injectable treatments in a single device (insulin+GLP-1 receptor agonist): insulin glargine plus lixisenatide, and insulin degludec plus liraglutide. Both have been shown to offer potent HbA1c reductions (1.6–1.9%), good behavior regarding body weight, a low hypoglycemia rate, and insulin dose savings compared to each component administered alone. They also represent a simple and convenient treatment option for the patient.64,65 However, they are not currently available in Spain.
Type 2 diabetes drug therapy algorithm of the SED 2018Fig. 1 seeks to embody the above considerations into a decision algorithm. What distinguishes the proposal of this consensus with respect to that of 2010 is that clearly stepwise treatment is now replaced by a more transverse decision strategy. Practically all the drugs are made available from the start with their salient differential features, thus allowing the prescribing physician to choose the combination option or options best suited to the needs of each individual patient. The aspects described above, such as a demonstrated reduction of cardiovascular risk, limitations on their use in renal failure, nephroprotection or complexity are all addressed in the table of available antidiabetic agents. With these novel aspects, a total of 9 characteristics are used to describe each drug class: efficacy (expected HbA1c reduction), the risk of hypoglycemia (including assessment of its severity, particularly applicable to sulfonylureas), the limitation on use in the event of renal failure, nephroprotection, the effects on body weight, the frequency of side effects (with mention of those most characteristic of each drug class, and with further information being provided in the text), the demonstrated impact upon cardiovascular risk, and the complexity and cost (taking into consideration pricing in the Spanish healthcare system). When the assessment is only applicable to one or more specific members of the drug class, a note in the text is provided. Each of these elements has a color code and is quantitatively described using a scale of that color.
Fig. 2 of the algorithm details the combination options of the different drug classes. There is no perfect or single combination for all patients. We therefore need to evaluate which combination is best suited to each individual patient. Combining DPP-4 inhibitors with GLP-1 receptor agonists is not advised.
Lastly, Fig. 3 details the start and adjustment of the available injectable therapies and their combinations: insulin (basal, rapid and premixed) and GLP-1 receptor agonists.
Due to the many cardiovascular safety studies that still need to produce results and the frequent incorporation of new treatments, the present recommendations will be updated with the indicated regularity.
Financial supportThe Consensus and Clinical Guidelines Working Group of the SED receives no sponsorship for carrying out its routine functions, and does not engage in activities subsidized by the pharmaceutical industry. Likewise, the Working Group has not received funding for developing this document.
Conflicts of interestThe authors state that they have no conflicts of interest in relation to the preparation of this document.
Please cite this article as: Gomez-Peralta F, Escalada San Martín FJ, Menéndez Torre E, Mata Cases M, Ferrer García JC, Ezkurra Loiola P, et al. Recomendaciones de la Sociedad Española de Diabetes (SED) para el tratamiento farmacológico de la hiperglucemia en la diabetes tipo 2: Actualización 2018. Endocrinol Diabetes Nutr. 2018;65:611–624.