Massilia timonae was described for the first time in 1998 by La Scola et al. as a fastidious, slowly growing, gram-negative bacterium.1M. timonae was isolated from a blood culture of an immunocompromised patient.
Since then, only six other cases of human infections caused by this microorganism have been described in the literature: three patients with sepsis, two with wound and bone infection, and the last one with a generalized lymphadenopathy and malaria coinfection. Only two of these patients had a relevant underlying condition.2,3,4 Herein, we describe the first isolation of M. timonae in ocular samples of a healthy woman with a corneal abscess.
Thirty-one year old female, with no history of systemic or ocular disease of interest, arrived to our hospital due to pain and photophobia in the right eye during the last two days. The patient referred neither systemic treatment, usage of contact lenses nor previous trauma. Visual acuity was 20/20 in both eyes. In the examination of the anterior pole with the biomicroscopy, erythema, moderate ciliary injection, two corneal abscesses with dense corneal stromal infiltrate and indistinct borders with 1mm approximately size, surrounding leukocyte infiltration and edema were observed. Both lesions had epithelial defect with positive fluorescence staining. No anterior chamber reaction was found. She preserved pupillary reflexes. Fundus and intraocular pressure was normal.
Corneal scraping with a hypodermic needle was performed, and the sample was sent to the microbiology laboratory. After that, empirical treatment with ciprofloxacin eye drops (3mg/ml Q2h) alternating with tobramycin eye drops (3mg/ml Q2h), night ointment of ciprofloxacin (3mg/g), and cyclopentolate eye drops (10mg/ml Q12h) was initiated.
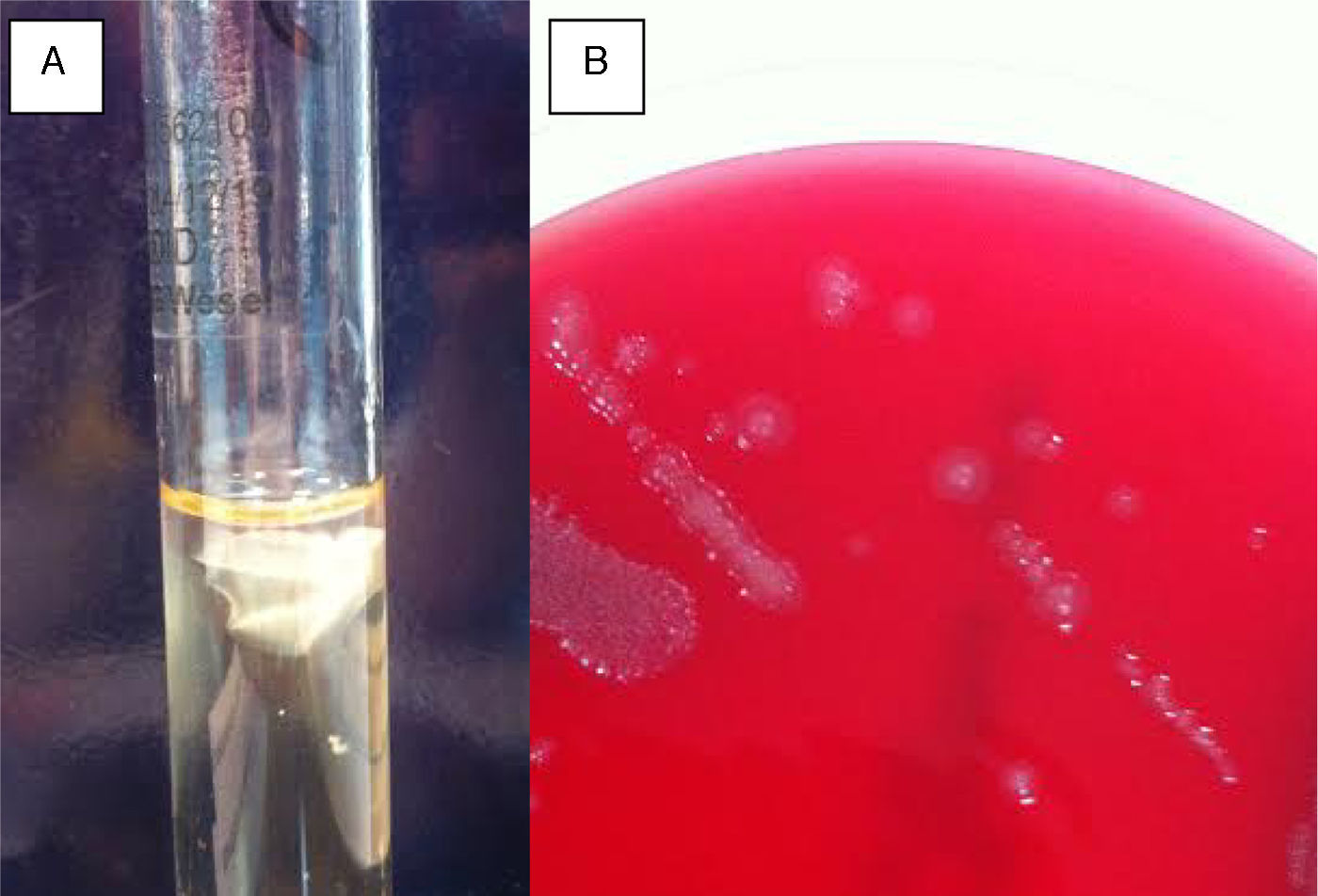
In the gram stain of the ocular sample, gram-negative rods were observed. The sample was cultured in blood agar, thioglycolate at 37°C and Sabouraud agar at 30°C. After 24-hour incubation, 2-3mm flat, shiny, greyish coloured colonies were observed in blood agar. In thioglycolate medium, a white layer inside the aerobic zone was observed. (Fig. 1)
A MALDI-TOF (Bruker-Daltonics, Bremen, Germany) with 24 grow blood agar colony was performed, and the result was M. timonae. Catalase and motility test were positive, while the oxidase test was negative. Moreover, API20NE test (bioMérieux, Marcy l’Etoile, France) was performed and the following results: reduction of nitrates to nitrites, indole production and glucose fermentation negative, and esculin and gelatine hydrolysis positive. It utilized as sole carbon source arabinose, mannose, maltose, malate, trisodium citrate and phenylacetic acid, but not manitol, N-acetyl-glucosamine, potassium gluconate, capric acid and adipic acid. The only discrepant test with La Scola et al. was arginine dihydrolase, which was negative; this difference had been previously in four strains of M. timonae.1,2
Bacterial identification was confirmed by DNA sequencing of the 16SrDNA. The DNA was extracted and PCR was performed with the following specific primers to amplify the 16S rRNA gene-coding region: 27F (5′AGA GTTTGA TCC TGG CTC AG 3′) and 1492R (5’ GGT TAC CTTGTT ACG ACT T 3′). PCR was performed using the following program: 94°C for 5min, followed by 30 cycles of 94°C for 40 s, 55°C for 40 s, 72°C for 80 s, and a final extension period of 72°C for 7min. 16S rDNA PCR products were sequenced and showed a 99% identity respect to that of the Massilia timonae strain 99A9205 (AY157761.1, GenBank).
The patient was re-examined within 24hours, with significant subjective improvement. The two abscesses persisted with less infiltration and resolution of the epithelial defect. Given the objective and subjective improvement the same treatment was kept, except for cyclopentolate eye drops.
The antibiogram was performed twice, using a commercial microdilution (NM44 MicroScan, Siemens, USA) and no growth was observed. The antibiogram was then performed with Etest on Mueller Hinton agar plates containing 5% sheep blood (Oxoid, Basingstoke, United Kingdom). MICs values were: ampicillin <0.016μg/ml, piperacillin-tazobactam 16μg/ml, amoxicillin-clavulanic acid 2μg/ml, clindamycin 0.125μg/ml, ceftazidime 0.0125μg/ml, ertapenem 0.25μg/ml, meropenem 0.016μg/ml, ciprofloxacin 0.064μg/ml, gentamycin 2μg/ml, tobramycin 2μg/ml, and trimethoprim-sulfamethoxazole 0.003μg/ml. Since there are not clinical breakpoints for this microorganism, MICs values were considered as susceptible according to CLSI breakpoints for non-Enterobacteriaceae.
A week later the patient continued improving. fluorometholone (2.5mg/ml) eye drops Q6h were added and frequency of antibiotics administration was decreased to 3mg/ml Q6h. A week later, there was a complete remission of symptoms and the treatment was concluded.
The genus Massilia belongs to the family Oxalobacteraceae (Betaproteobacteria), and up to now comprises twenty-five species.5 Members of the genus are characterized as aerobic, gram-negative and non-spore forming rods. This environmental microorganism has been previously described as the main cause of infection in humans in other samples, such a cerebrospinal fluid, blood, soft tissues and bone, but this is the first case where M. timonae is isolated from cornea.5,6,7,8
Despite the lack of virulence of this microorganism and the absence of resistance to the majority of antibiotics, it is important to achieve a correct identification of that pathogen in all types of samples. M. timonae has proved to cause infection in different locations, independently of the underlying condition of the patients. Due to all these reasons, correct isolation and identification should routinely be performed.
In summary, this is the first case described of corneal abscess caused by M. timonae in a healthy woman.