We report the case of an 82-years-old woman moderately underweight (weight, 34.4kg; BMI, 16.8kg/m2), one kidney patient who was admitted to hospital for presenting fever and abdominal pain. At admission, a hydatidic cyst with calcification and fistulization to a new perihepatic collection was observed. Ten hours later, the patient was moved to the intensive care unit (ICU) due to severe hypotension and no response to the administration of fluids. Serum creatinine increased from 250.07μmol/L to 365.98μmol/L, and glomerular filtration rate (GFR) decreased from 0.28mL/s/m2 to 0.18mL/s/m2.
On the same day, a turbid fluid collection from the peritoneal drainage appeared and oral albendazole 400mg twice daily, intravenous imipenem 500mg twice daily and intravenous linezolid (LZD) 600mg twice daily (34.9mg/kg/day) were prescribed. After 5 days of treatment, her clinical situation improved without normalization of renal function (GFR 0.23mL/s/m2). However, a decrease of 48.6% in the platelet count was observed and the patient experienced a mild hand tremor.
Due to suspicion of a possible overexposure to LZD, plasma concentrations were measured and a trough (pre-dose) and peak (at the end of 1h infusion) samples were obtained. Trough and peak concentrations of LZD were 52.1mg/L and 93.2mg/L, and LZD treatment was stopped. Serial blood samples were obtained at 24, 48, 72, 96, 120 and 144h after the discontinuation of LZD.
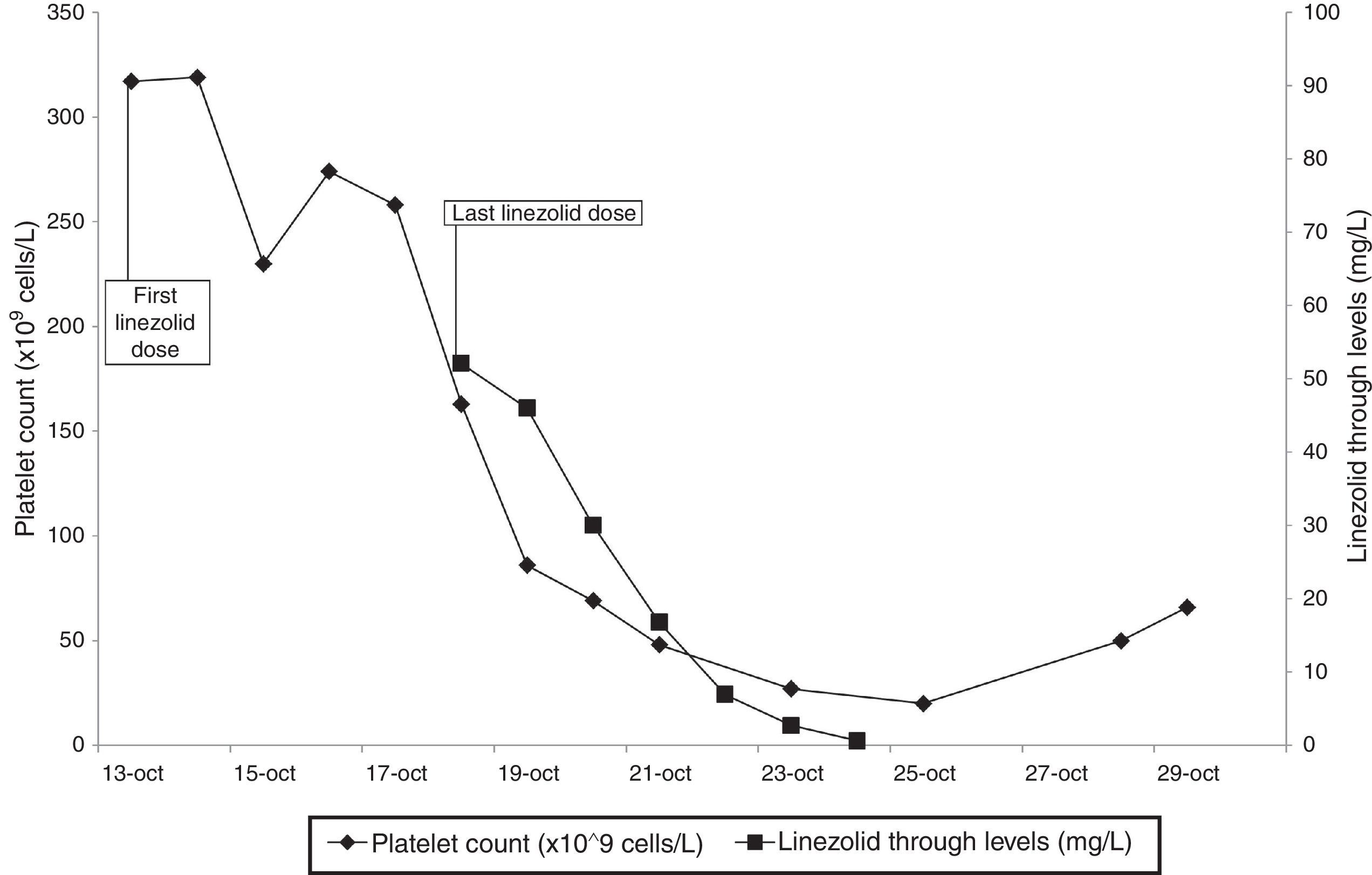
Linezolid plasma levels and platelet count are shown in Fig. 1.
Patient's serum haemoglobin also decreased from 129g/L at the start of the LZD treatment to a nadir value of 88g/L at the 11th day after the LZD discontinuation. An inverse lineal correlation was observed between the platelet count and LZD plasma levels (Pearson's correlation coefficient: −0.893 (p=0.041) but not with haemoglobin. The patient did not receive any blood nor platelet transfusion.
On 10th day of ICU admission, patient's clinical evolution improved and she was moved to a surgery ward. However, 7 days after, she was diagnosed with a septic shock due to a complicated intra-abdominal infection. The patient did not respond to fluid resuscitation and inotropic drugs and finally died 24h later.
LZD is commonly used in the treatment of infections caused by multidrug-resistant Gram-positive cocci in critically ill patients. In this population, due to a high variability in the LZD pharmacokinetics, therapeutic drug monitoring (TDM) has been recommended.1,2
LZD use has been related to haematological toxicity (thrombocytopenia, anaemia), neuropathy (peripheral and optical), lactic acidosis and gastrointestinal disturbances.3,4 Different risk factors of haematological toxicity, which is dose-dependent and reversible, have been identified: age, baseline platelet count <200×109cells/L, renal dysfunction, low body weight, treatment >14 days and chronic liver disease, being two of them present in our patient.3–5
LZD dose is not usually reduced in patients with renal impairment, although those are more likely to achieve high plasma concentrations.3,5 LZD is metabolized into two major inactive metabolites (PNU-142586 and PNU-142300) which are primary renal excreted.3,5 It remains unknown the potential effect of their accumulation but they may potentiate the haematological toxicity of LZD.
Low body weight has been described as a risk factor for LZD-induced thrombocytopenia. A dose adjustment of 20mg/kg/day in patients weighting <55kg seems to be effective in preventing this side effect.4 Our patient weighted 34.4kg, so a dose of 350mg/12h should have been administered. Nevertheless, a fixed dose of 600mg/12h is routinely prescribed, independently of the patient's body weight.
Regarding the plasma through level of LZD, it was 7-fold higher than 7.5mg/L, value identified as a predictor of LZD-induced thrombocytopenia.3
The possible effect of albendazole in the haematological toxicity seems unlikely because she previously received two cycle treatments without alterations in the haematological parameters.
Although the mild hand tremor was not a clinically relevant side effect, the high levels of LZD may have had an influence.
To our knowledge, this is the case with the highest value of LZD plasma levels ever reported, which was probably caused by an overdosing in a patient with moderate underweight and severe renal dysfunction. The administration of a weight-based dose-adjustment and TDM should be considered in patients presenting these physiopatological conditions to ensure efficacy while preventing overexposure and toxicity.
FundingThe study was carried out as a part of our routine work.
Conflict of interestThe authors declare no conflicts of interest.