The aim of this study was to compare the colonization rates of central venous catheter (CVC) and arterial catheter (ArtC) hubs fitted with two types of needleless connectors (NCs).
MethodsWe designed a prospective randomized study to compare rates of catheter hub colonization of CVC and ArtC hubs fitted with two types of needleless connectors: neutral-pressure NCs (NP-NCs) and positive-pressure NCs (PP-NCs) in critically ill patients. All NCs were replaced every 7 days of use.
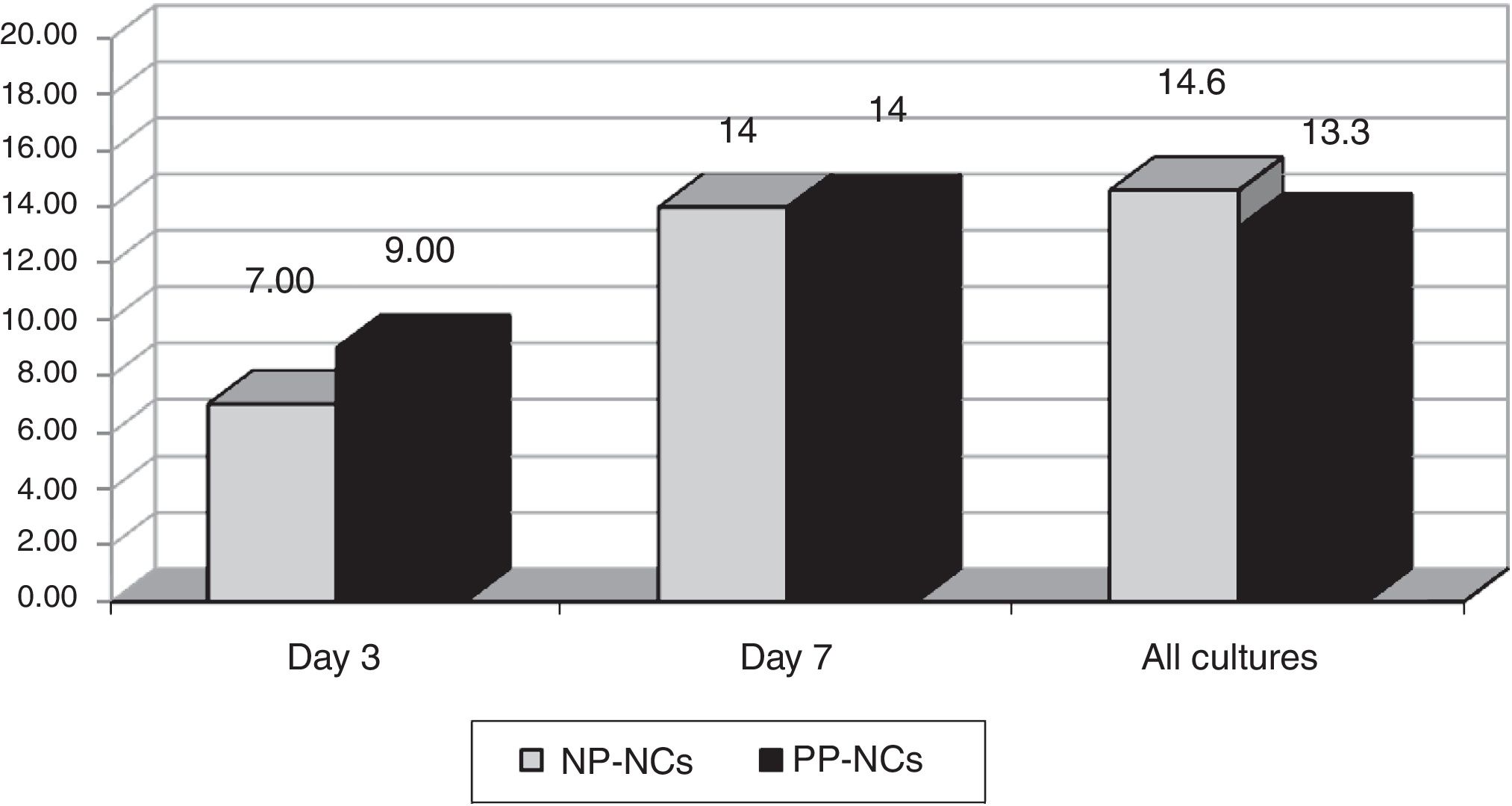
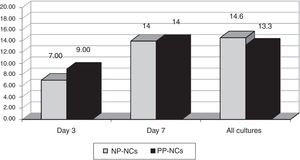
ResultsWe obtained 326 cultures from 146 catheters (81 CVC and 65 ArtC) in 70 patients. The total cumulative days of risk were 1250 catheter-days. Global swab cultures were positive in NP-NCs in 29/198 (14.6%) versus 17/128 (13.3%) in PP-NCs during catheter use. We did not observe any cases of CRBSI.
ConclusionsIn our experience, the use of PP-NCs did not result in significantly more frequent hub colonization with respect to NP-NCs.
El objetivo de este estudio fue comparar las tasas de colonización de las conexiones de catéteres venosos centrales (CVC) y catéteres arteriales (ArtC) equipados con 2 tipos diferentes de conector desinfectable (CD) en pacientes críticos.
MétodosRealizamos un estudio aleatorizado prospectivo. Los 2 tipos de conectores desinfectables comparados fueron un conector de presión neutra (CDPN) y un conector de presión positiva (CDPP). Todos los CD fueron reemplazados cada 7 días de uso.
ResultadosObtuvimos 326 frotis de las conexiones de 146 catéteres (81 CVC y 65 ArtC) en 70 pacientes, con un total de días de riesgo de 1.250. Los cultivos fueron positivos en 29/198 (14,6%) de los CDPN respecto a 17/128 (13,3%) en los CDPP (p: NS). No hubo ningún caso de bacteriemia por catéter.
ConclusionesEn nuestra experiencia, el uso de CDPP no supone un aumento en la tasa de colonización de las conexiones respecto a los CDPN.
The role of needleless connectors (NCs) and particularly their design in the pathogenesis of CRBSI has been a matter of controversy.1–4 Some experimental models have analyzed the efficacy of various connectors against contamination under different conditions of handling or daily clinical practice.5–7 Experimental models have also shown clear differences in colonization rates between different types of NCs.1,2
The aim of this study was to compare the rates of colonization of central venous catheter (CVC) and arterial catheter (ArtC) hubs fitted with two types of NCs: neutral pressure NCs (NP-NCs) and positive pressure NCs (PP-NCs) in critically ill patients.
MethodsA prospective randomized clinical trial was carried out in a 14-bed polyvalent intensive care unit (ICU) at the Hospital de Mataró (Barcelona, Spain). The inclusion criteria were: (1) patients older than 18 years, (2) catheter insertion performed by strictly sterile procedure in the surgery area or ICU of our centre within the previous 24h, (3) informed consent for participation by patients or their legal representatives. The exclusion criteria were catheter insertion in another centre, catheter insertion for more than 24h before randomization or catheter insertion over a guidewire. Patients were consecutively included at ICU admission and randomly assigned to one of the two catheter groups by the sealed envelope procedure. In one group, catheters were fitted with a PP-NC (SmartsitePlus Carefusion, San Diego, CA), and in the other group with a NP-NC (MicroClave ICU Medical, San Diego, CA).
Catheters were inserted and manipulated according to our hospital protocol, based on the CDC HICPAC (2006) recommendations,8 in force at the time of the study. The infusion system and NCs were assembled under sterile conditions at the time of insertion and were replaced every 7 days. Infusion lines used to infuse lipid solutions or blood products, were changed every 24h.8,9 The attending ICU nurses were trained in catheter management and were highly aware of the requirement to thoroughly disinfect the connectors with a cellulose wipe and 0.5% alcoholic chlorhexidine before and after their use. The pressurized line in ArtC and the distal infusion system in CVCs were temporarily disengaged using a strictly sterile technique to enable swabbing of the catheter hub. An alginate swab was pushed into the hub and rotated from 3 to 5 times in its interior. This procedure was carried out in each patient using maximal sterile barriers (surgical cap, face mask, sterile gloves, and sterile gown) to avoid contamination in sample collection. Samples processed from the catheters were directly related to the time the catheter was in use. Cultures were made every day 3 and 7 of each week of use and before the periodical NCs replacement. Therefore, a catheter inserted 11 days, generated 3 cultures (day 3, 7, 10).
The main outcome measures were colonization of the hub, defined as more than 15 CFU of bacterial growth on hub swab culture, and catheter-related bloodstream infection, established on the isolation of the same microorganism in semiquantitative catheter tip culture and blood culture, with no evidence of an alternative infectious focus.8–10 The decision to obtain blood cultures and catheter withdrawal for diagnosing CRBSI was at the discretion of the attending physician according to protocol in our centre. All catheters removed for CRBSI suspicion were cultured. The study lasted 5 months.
Calculation of sample size and statistical analysisPrevious clinical trials comparing NCs and systems using conventional caps yielded connector colonization rates in the NCs group from 4.3% to 28% and CRBSI rates from 0.7% to 9%.11 The sample size required to detect a difference in the hub colonization rate of 5% in the best connector to 20% in the worst, with an alpha error of 0.05 and a beta error of 0.10, would be 68 cultures per NCs group. In the statistical analysis, 2×2 contingency tables were used to assess differences between discontinuous variables. Significance was set at a p value of ≤0.05.
95% CI was calculated to compare non-independent variables.
The study was evaluated and approved by our institutional ethical committee.
All persons gave their informed consent prior to their inclusion in the study.
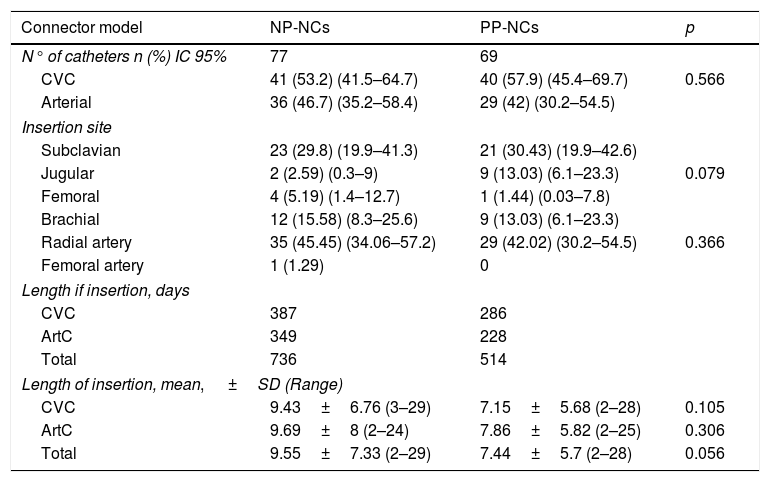
ResultsThe study included 70 patients with a mean age of 69 years (25–92). There were no statistically significant differences between the groups with respect to demographic characteristics, the reason for admission, SPAS II score or risk factors for infection, as mechanical ventilation, nasogastric tube, urinary catheter, enteral nutrition, total parenteral nutrition, antibiotic therapy, or corticosteroid treatment. A total of 81 central venous catheters and 65 arterial catheters were used in the randomized patients. Total days of risk (total catheter-days) was 1250. Catheter-related characteristics in the two catheter groups are set forth in Table 1.
Catheter-related characteristics in the two catheter groups.
| Connector model | NP-NCs | PP-NCs | p |
|---|---|---|---|
| N° of catheters n (%) IC 95% | 77 | 69 | 0.566 |
| CVC | 41 (53.2) (41.5–64.7) | 40 (57.9) (45.4–69.7) | |
| Arterial | 36 (46.7) (35.2–58.4) | 29 (42) (30.2–54.5) | |
| Insertion site | |||
| Subclavian | 23 (29.8) (19.9–41.3) | 21 (30.43) (19.9–42.6) | 0.079 |
| Jugular | 2 (2.59) (0.3–9) | 9 (13.03) (6.1–23.3) | |
| Femoral | 4 (5.19) (1.4–12.7) | 1 (1.44) (0.03–7.8) | |
| Brachial | 12 (15.58) (8.3–25.6) | 9 (13.03) (6.1–23.3) | 0.366 |
| Radial artery | 35 (45.45) (34.06–57.2) | 29 (42.02) (30.2–54.5) | |
| Femoral artery | 1 (1.29) | 0 | |
| Length if insertion, days | |||
| CVC | 387 | 286 | |
| ArtC | 349 | 228 | |
| Total | 736 | 514 | |
| Length of insertion, mean, ±SD (Range) | |||
| CVC | 9.43±6.76 (3–29) | 7.15±5.68 (2–28) | 0.105 |
| ArtC | 9.69±8 (2–24) | 7.86±5.82 (2–25) | 0.306 |
| Total | 9.55±7.33 (2–29) | 7.44±5.7 (2–28) | 0.056 |
NP-PC: neutral pressure connector, PP-NC: positive pressure connector, CVC: central venous catheter, ArtC: arterial catheter, SD, Standard deviation.
We obtained 198 cultures in the NP-NC and 128 in the PP-NC, total of 326. Colonization rates observed are shown in Fig. 1. No differences in hub colonization rates were observed. The most frequent bacteria isolated were coagulase-negative staphylococci. No cases of CRBSI were detected in either group during the study period.
DiscussionVery few prospective studies have analyzed the true impact of the type of connector on the rate of infections in clinical situation before ours. In this prospective randomized study, no significant differences were found in the catheter hub colonization rates associated with two different disinfectable NCs systems. In our setting, the risk of hub colonization was not higher with the PP-NCs than with the NP-NCs.
A major subject of discussion on CRBSI prevention focuses on the safety of different connector designs, mainly those designed to generate a positive pressure. In a prospective study performed in a paediatric population, the use of positive pressure connectors was associated with a higher risk of developing CRBSI.4 However, in another study that compared also NP-NCs to a different model of PP-NCs, the CRBSI rate was reduced significantly.12 The reasons for these associations are unknown and it is also not known if this is secondary to physical or mechanical properties of NCs, which vary from device to device. Some studies have shown that the increase in CRBSI rates with the change to luer-activated devices may be related to improper cleaning of NCs.13 The need to disinfect the connectors before its use requires healthcare worker training,14 which has been interpreted as a limitation of these devices. One experimental model illustrated the importance of disinfection to prevent permeability of connectors to microorganisms, even above and beyond the differences in design.3 In our ICU, connector disinfection is one of the major points covered in the institutional guidelines for endovascular catheter management. Correct handling is essential when NCs are used. The absence of differences between the two connector models used in this study could be attributed in part to close adherence to catheter management recommendations by the health staff.
Our study has several limitations as follows. The low incidence of CRBSI hinders the development of prospective randomized trials. The hub colonization rate could be considered a minor objective. However, clinical, microbiological, and electron microscopy studies have indicated that the hub is an important source of colonization and CRBSI2,10 and it is only slightly affected by factors other than connector handling.
The aim of our study was to compare catheter hub colonization rates and not CRBSI rates which could be understood as a limitation. However, we understand that this allows us to evaluate the safety of the connector design properly, avoiding other confusing factors that can be involved in CRBSI appearance. We did not perform catheter tip cultures. Performing systematic tip culture on all of the removed catheters would have involved monitoring all catheters not removed at discharge from the ICU, which could be manipulated differently in other areas of the hospital. On the other hand, removing the still necessary catheters would have involved an ethical conflict. For this reason, the catheters were monitored clinically for 72h after discharge from the ICU. None of the patients with CVC or ArtC subsequently presented any episode of catheter-related bacteremia. In the same way, it was not considered an objective of our study to determine the colonization rate of the patient's skin, given that the study has a very specific objective related to the manipulation and safety of the connectors. In our experience, the use of PP-NCs did not result in significantly more frequent colonization of central venous or arterial catheter hubs with respect to the use of NP-NCs.
In conclusion colonization of NCs may depend more on the incorrect handling and NC design rather than on the pressure.
Conflict of interestsDr. Yébenes has received honoraria from Carefusion for consultancy and speaker fees from Carefusion and ICU Medical.
The other authors declare that they have no conflict of interest.