There are very few data available regarding risk factors associated with antibiotic resistant-Neisseria gonorrhoeae.
MethodsA study was conducted on 110 samples from 101 patients with gonococcal infection, in order to describe their characteristics and compare them with the antimicrobial susceptibility profile of their samples.
ResultsAn association was observed between resistant infections and heterosexual men, older age, concurrent sexually transmitted infection, and unsafe sexual behaviors.
ConclusionThere is a need for improved data on the risk factors associated with antibiotic resistant gonococcal infection in order to identify risk groups, and to propose public health strategies to control this infection.
: Disponemos de poca información sobre los factores de riesgo asociados a la infección por gonococo resistente a antibióticos.
MétodosEstudiamos 110 muestras de 101 pacientes, describimos sus característica y las comparamos según el perfil de susceptibilidad antimicrobiana de sus muestras.
Resultadosobservamos una asociación significativa entre las infecciones por cepas resistentes y varones heterosexuales, edad avanzada, infección de transmisión sexual concurrente y comportamientos sexuales de riesgo.
Conclusioneses necesario ampliar el estudio sobre los factores de riesgo asociados a infecciones por gonococo resistente con el objetivo de implementar medidas estratégicas para controlar la infección.
Sexually transmitted infections (STI) caused by Neisseria gonorrhoeae (NG) present a high global incidence and significant associated morbidity. Furthermore, NG has developed resistance to all antibiotics prescribed for its treatment.1 In the last years treatment failures with third generation cephalosporins have been reported which may lead to untreatable gonorrhea in the near future.1 In fact, the latest report from the European Gonococcal Antimicrobial Surveillance Programme (Euro-GASP, 2013) identified a high proportion (4.7%) of NG isolates with decreased susceptibility to cefixime and 7 isolates with decreased susceptibility to ceftriaxone. Rates of ciprofloxacin, penicillin (high-level resistance) and azithromycin resistance were also too high (53%, 12.9% and 5.4%, respectively) to recommend as first-line therapies.2 However, few data regarding risk factors associated with antibiotic resistant-NG are available in Europe.3 Our aims were to study the susceptibility of NG isolates obtained in our center and determine a possible link between epidemiological and behavioral characteristics of patients infected and the antimicrobial susceptibility profile of their NG strains.
Materials and methodsStudy designWe conducted a retrospective cross sectional study among patients recruited in a tertiary care hospital in Barcelona (Spain), stratified by HIV status. Most of them attended the STIs Unit. This unit works as a walk-in clinic in the downtown part of the city. We reviewed data from all patients with NG isolates obtained from June 2009 to December 2014 for which susceptibility study was done (n=286) but included only patients with recorded socio-demographic, clinical and behavioral parameters.
Data were collected from the Barcelona STI registry and handled on a strictly confidential basis according to the requirements of the Spanish data protection law. The research protocol was approved by the Research Ethics Committee of our center.
Statistical analysisData were analyzed using the statistic package Stata 13.0 (Statacorp®Texas, USA). Means, medians, standard deviation (SD) and proportions were used as necessary for the descriptive analysis. Confidence intervals of 95% were considered for proportions. Student t test or Chi-square were used for statistical analysis, or their non-parametric counterparts when necessary (Wilcoson or Fisher exact test). For multivariate analysis, logistic regression was used. A p value ≤0.05 was considered significant.
Sample collection and laboratory methodsUrethral, endocervical, rectal and pharyngeal swabs were inoculated onto chocolate and Thayer-Martin agar. Susceptibility to antibiotics was determined by the E-test and detection of beta-lactamase by nitrocefin test, following the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines and breakpoints.4 58 samples (52%) detected since December 2012 were also tested for azithromycin. Strains with susceptibility to ciprofloxacin, 3rd generation cephalosporins (cefixime and cefotaxime) and penicillin and those strains with only low resistance to penicillin (negative beta-lactamase production and intermediate susceptibility for PBPs modification) were considered as not antimicrobial resistant (NAMR) NG.
ResultsFrom June 2009 to December 2014, 286 NG isolates were obtained. We included 110 samples of NG infection for which epidemiological and behavioral data were available.
These samples were from 101 patients. Five patients suffered 2 NG infections, and 2 patients 3 different events. All these cases were sufficiently separated in time to consider them as independent infections.
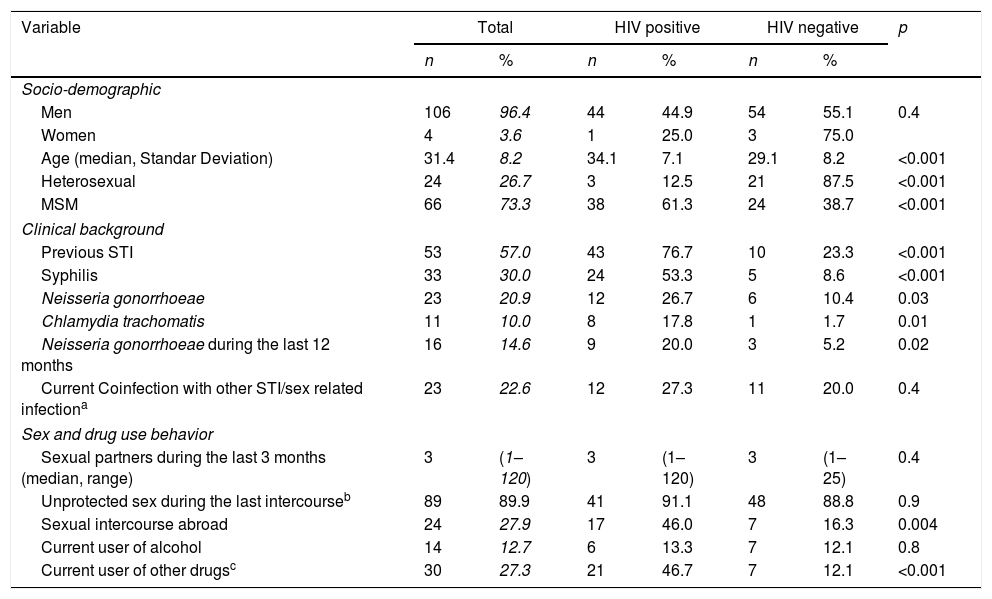
Table 1 shows the socio-demographic, clinical, and behavioral data of the cases included. Mean age was 31.4 years and 96.4% (n=106) were male. Men who have sex with men (MSM) represented 73.3% (n=66) of patients and 41% (n=45) were infected with human immunodeficiency virus (HIV). Samples were collected from urethral 85.9% (n=98), 8.7% rectal (n=10), 1.7% pharyngeal (n=2), and 2.6% vaginal sites (n=3). When comparing characteristics of patients according to their HIV status (see Table 1), we found that among our patients with gonococcal infection, concomitant HIV infection was significantly associated with age (7.1% vs 8.2%, p<0.001), MSM (61.3% vs 38.7%, p<0.001) and history of previous STI (76.7% vs 23.3%, p<0.001). Risky sexual behavior such as sexual activity in foreign countries (46.0% vs 16.3%, p=0.004) and use of cocaine (24.4% vs 3.5%, p=0.001) or cannabis (20.0% vs 5.2% p=0.02) before sexual intercourse were also significantly associated with HIV infection.
Socio-demographic, clinical, behavioral, data and characteristics of patients according to their HIV status.
| Variable | Total | HIV positive | HIV negative | p | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Socio-demographic | |||||||
| Men | 106 | 96.4 | 44 | 44.9 | 54 | 55.1 | 0.4 |
| Women | 4 | 3.6 | 1 | 25.0 | 3 | 75.0 | |
| Age (median, Standar Deviation) | 31.4 | 8.2 | 34.1 | 7.1 | 29.1 | 8.2 | <0.001 |
| Heterosexual | 24 | 26.7 | 3 | 12.5 | 21 | 87.5 | <0.001 |
| MSM | 66 | 73.3 | 38 | 61.3 | 24 | 38.7 | <0.001 |
| Clinical background | |||||||
| Previous STI | 53 | 57.0 | 43 | 76.7 | 10 | 23.3 | <0.001 |
| Syphilis | 33 | 30.0 | 24 | 53.3 | 5 | 8.6 | <0.001 |
| Neisseria gonorrhoeae | 23 | 20.9 | 12 | 26.7 | 6 | 10.4 | 0.03 |
| Chlamydia trachomatis | 11 | 10.0 | 8 | 17.8 | 1 | 1.7 | 0.01 |
| Neisseria gonorrhoeae during the last 12 months | 16 | 14.6 | 9 | 20.0 | 3 | 5.2 | 0.02 |
| Current Coinfection with other STI/sex related infectiona | 23 | 22.6 | 12 | 27.3 | 11 | 20.0 | 0.4 |
| Sex and drug use behavior | |||||||
| Sexual partners during the last 3 months (median, range) | 3 | (1–120) | 3 | (1–120) | 3 | (1–25) | 0.4 |
| Unprotected sex during the last intercourseb | 89 | 89.9 | 41 | 91.1 | 48 | 88.8 | 0.9 |
| Sexual intercourse abroad | 24 | 27.9 | 17 | 46.0 | 7 | 16.3 | 0.004 |
| Current user of alcohol | 14 | 12.7 | 6 | 13.3 | 7 | 12.1 | 0.8 |
| Current user of other drugsc | 30 | 27.3 | 21 | 46.7 | 7 | 12.1 | <0.001 |
MSM: men who have sex with men; STI: sexually transmitted infections.
Unprotected sex before symptoms appeared in 88.1% (n=89) of the patients and drug use before having sex within the last 12 months before diagnosis in 48.2% (n=42). Coinfection with other STI (different from HIV) at the time of diagnosis was found in 22.6% (n=24) while 57.0% (n=53) had been previously diagnosed with another STI (see Table 1).
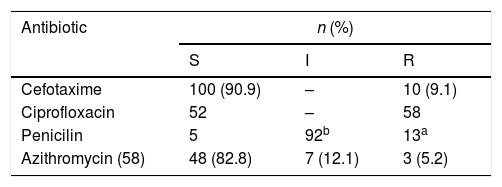
Susceptibility studyOnly 44% (n=49) of NG isolated strains were sensitive to all the antibiotics tested (most of them (89.7%) with intermediate susceptibility to penicillin due to PBPs modification). Ciprofloxacin resistance was found in 52.7% (n=58) of isolated strains, 11.81% (n=13) strains showed high penicillin resistance (induced by production of beta-lactamase), 9.1% (n=10) showed resistance to 3rd generation cephalosporins (with MICs of cefotaxime ranging from 0.19 to 0.75 mcg/ml), of 58 strains tested to azithromycin, 5.2% (n=3) showed resistance and 12.1% (n=7) intermediate susceptibility. No resistance to gentamicin or spectinomycin was detected.
Risk factors associated with caring a N. gonorrhoeae resistant strainFor gonococcal isolates that were ciprofloxacin resistant, there was a significant protective association in the adjusted analysis with infection in MSM (OR=0.30, p=0.03) and a risk factor for older patients (OR=1.46, p=0.03). Isolates that were cefotaxime resistant presented a significant crude protective association with HIV positive patients (OR=0.12, p=0.05). Individuals with a higher number of sexual partners in the previous 3 months were most prone to carry a cefotaxime resistant strain (p=0.008), as well as older patients (OR 2.13, p=0.05), those who reported recent sexual intercourse in a foreign country (OR 26.9, p=0.02) and those coinfected with another STI (OR=29.9, p=0.02). Older patients (OR 1.47, p=0.04) and previous gonorrhea in the last 12 months (OR=4.92, p=0.04) were independent factors associated with carriers of AMR NG strain (see Table 2).
In vitro susceptibilities of Neisseria gonhorroeae isolates (E-test or disk diffusion, EUCAST) (S=sensible, I=intermediate, 3=resistant, n=number of isolates).
| Antibiotic | n (%) | ||
|---|---|---|---|
| S | I | R | |
| Cefotaxime | 100 (90.9) | – | 10 (9.1) |
| Ciprofloxacin | 52 | – | 58 |
| Penicilin | 5 | 92b | 13a |
| Azithromycin (58) | 48 (82.8) | 7 (12.1) | 3 (5.2) |
1. Adjusted by age, HIV status and sexual orientation. 2. Adjustment was not possible because the value of one strata was 0, and all cefotaxime resistant strain carriers with data available (n=6) had higher than the median number of sexual intercourse episodes during the previous 3 months. 3. Age has been considered as continuous variable using the following groups: <20, 20–24, 25–29, 30–34, 35–30 and ≥40. 4. During the previous 12 months (n=16).
A particular finding from our study sample of NG infection cases is the high prevalence of foreign patients, MSM, patients engaging in unsafe sexual behavior and HIV patients compared to previous studies on these topics.3 A recent official report on gonococcal surveillance in our region5 observed that 85% of infections occurred in men (compared to 96.4% in our study); 46% were MSM (73.3% in our study); 19% were HIV+ (41% in our study), and 26% were born out of Spain (41% in our study). Our STI Unit is part of a tertiary care hospital with an important HIV department, located in the downtown area of a large city. The origin of our patients may explain the lack of representativeness of the general population. However, we consider that these differences do not affect the main findings of the study.
In our unit we attend patients with symptomatic STI, which explains that most of our samples were collected from urethral site as most cases of pharyngeal and anorectal NG infection are asymptomatic.
One striking point is the significant increase of STI incidence in MSM with a high HIV coinfection rate, a worrisome problem previously reported in our city,6 corroborated by the high incidence of non-protected sex reported among patients included in our study. In fact, MSM as a vulnerable population for STI and HIV infection in major European cities is currently a primary concern to public health.7
Our data showed a significant association between heterosexual men and being a carrier of ciprofloxacin resistant or any AMR strains. Recent studies have linked AMR gonococcal infections with both heterosexual and MSM networks across Europe and USA.2,3,8 An association between AMR gonococcal infections and heterosexual men in European countries has been recently reported.3 We consider that associations between AMR isolates and HIV are likely confounded by MSM.
Our results also reveal an association between AMR to 3rd generation cephalosporins and unsafe sexual behavior. Several studies have previously linked AMR-NG infection with risky sexual behavior such as sex with sex workers and recent foreign travel.8 Concurrent STI was also associated with resistance to cefotaxime. We can presume that patients engaging in risky sex behavior and concurrent infections might have been previously been infected with others STI and therefore treated with antibiotics which could have led to the development of a resistant infection. Accordingly, carriers of AMR strains were associated with previous NG infection.
We also found a significant association between age and being carriers of ciprofloxacin and cefotaxime resistant isolates or any AMR strains which has previously been reported.3,7,9
Our main limitation is the relatively low number of patients included in the study. Selection and information bias may have been introduced by excluding patients from whom data were not available and by using confidential interviews as the data collection method. We consider that these limitations do not invalidate the main objective of the study which was to determine epidemiological and behavioral risk factors for acquiring AMR NG.
ConclusionsMost of our results support previous data recently reported on this issue2,3,7,8 including the European guidelines recommendation2,10 to avoid ciprofloxacin, azithromycin and penicillin as empirical treatment for gonorrhea in our patients. This study also underlines the need to implement effective interventions to control the extension of STI through core groups of MSM, especially those coinfected with HIV, in large cities of Western Europe.
We consider that improved data about risk factors linked with AMR gonococcal infection are required to identify evidence-based risk groups. Accessible and high quality STI services for these key groups are essential to control this infection.
Author contributionsDrs. Fuertes and Baliu had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Drs. Fuertes, and Bosch. Acquisition of data: Drs. Fuertes, Vergara and Alsina. Analysis and interpretation of data: Drs. Fuertes, Baliu and Vallés. Drafting of the manuscript: Drs. Fuertes, Alsina and Bosch. Critical revision of the manuscript for important intellectual content: Drs. Fuertes, Bosch and Alsina. Statistical analysis: Drs. Fuertes and Baliu.
Funding/SupportThis study was supported in part by the Hospital Clinic de Barcelona. The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; or in the preparation, review, or approval of the manuscript.
Financial disclosureNone reported.
Conflicts of interestNone declared.