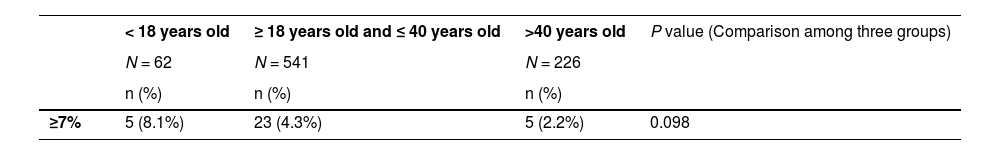Blonanserin is a second-generation antipsychotic (SGA)for the treatment of schizophrenia and has been approved for use in Japan, South Korea and China. This analysis aimed to evaluate the effectiveness and safety of blonanserin in Chinese adolescent patients, using data from a post-marketing surveillance of blonanserin started in September 2018.
MethodsA 12-week, prospective, multi-center, open-label post-marketing surveillance was conducted. Patients in this analysis were stratified by age.
Results78 patients with schizophrenia aged < 18 years were included. The incidence of adverse drug reactions in adolescent patients was 33.3% significantly higher than patients over 40 years old. After 12-week treatment, 8.1% of adolescent patients had a weight gain ≥ 7%, and it was not significantly different from other age groups. Mean Brief Psychiatric Rating Scale (BPRS) total score was significantly reduced at week 12, and the decline of anergia score and thought disturbance score of adolescent patients was significantly greater than in adults.
ConclusionsBlonanserin was well tolerated and effective in the treatment of schizophrenia in Chinese adolescent patients in real-world clinical practice. Blonanserin might be a reasonable choice for the treatment of schizophrenia in adolescent patients.
Schizophrenia in children and adolescents belongs to early-onset schizophrenia (EOS). As a subtype of schizophrenia, it is generally similar to the clinical presentation (i.e., positive symptoms, negative symptoms, functional deficits) of adult-onset schizophrenia (AOS).1 In addition, compared with AOS, children and adolescent patients often have atypical early symptoms and more prominent negative symptoms, which greatly affect the patient's social ability, cognitive function, and learning ability.2 Moreover, the frequency and duration of schizophrenia episodes have deleterious neuropsychological, neurophysiologic, and neurostructural effects, making rapid, aggressive, and effective treatment a key component of care.3
Atypical antipsychotic blonanserin was first approved by the Pharmaceuticals and Medical Devices Agency (PMDA) for the treatment of schizophrenia in 2008 and obtained approval from the Korean and Chinese medical administrations subsequently. It is a highly selective antagonist of dopamine D2, D3 and serotonin 5-HT2A receptors.4 A long-term extension study showed that oral blonanserin treatment improved or stabilized psychiatric symptoms in adolescents with schizophrenia.5 Another Japanese meta-analysis research, conducted in 2019, produced a favorable result on the safety of blonanserin.6 A randomized controlled study has shown that blonanserin was safe and well tolerated for adolescents, although they are usually more sensitive to psychotic drugs.7
The administrative approval of a newly marketed psychotic drug is a provisional subject, as the administration could discontinue or even terminate its legal status in consideration of public well-being. Thus, post-marketing surveillance (PMS) is adopted by regulators as essential means to conduct further inspections of newly developed drugs after they are marketed to the public on a scale.
The PMS of blonanserin in China was initiated in 2018, using a twelve-week non-interventional design, to observe blonanserin's safety file and effectiveness in patients with schizophrenia. To our knowledge, this is the first report on the safety and effectiveness of oral blonanserin for schizophrenia in adolescents in the Chinese population.
Patients and methodsPatientsThis multicenter, observative, non-interventional, and prospective, 12-week PMS study, was conducted from September 2018 to May 2020, at 16 sites throughout China. The study protocols were approved by the ethics committees of the leading clinical site at the Second Xiangya Hospital of Central South University and the respective other study centers. Written informed consent was obtained or a waiver of informed consent was approved by the clinical site where the patients were enrolled.
Concomitant drugsConcomitant drugs like antipsychotics, antidepressants, and antiepileptics were not limited during the whole surveillance. Besides satisfying the patient's medical needs, this also produced a fabulous opportunity to evaluate the tolerance of blonanserin in the presence of other medications. For scientific and safety purposes, all prescriptions would be documented in the electronic case report form (e-CRF).
Safety evaluationThe safety measurements included:
- 1)
The incidence of adverse event / adverse drug reaction (ADR)
- 2)
The proportion of patients who received combined medication by the end of the follow-up.
- 3)
The change in weight, in comparison to the baseline, by the end of the follow-up.
The effectiveness measurements included: the change of BPRS total score in comparison to the baseline, by the end of the follow-up.
Statistical analysisThis research used SAS Version 9.4. Categorical variables were shown as n (%) and continuous variables were summarized as mean ± standard deviation (SD). Paired t-test and analysis of variance (ANOVA) were used to compare continuous variables and χ2 analysis was used for categorical variables. The statistical significance level was defined with a two-tailed P-value of < 0.05.
In this study, the full analysis set (FAS) was used for the analysis of demographic data, baseline characteristics, and main efficacy. FAS includes all monitored patients who have used the monitored drug at least once. The safety set (SS) was used for the analysis of drug safety. SS includes the actual data that received at least one blonanserin treatment.
ResultsBaseline demographicsIn 1018 cases of FAS, the mean age was 32.9 ± 13.26 years old. 78 patients < 18 years from 10 clinical sites were included in this analysis. There were 78 (7.66%) cases that were adolescents, with an average age of 16.1 ± 1.48 years old. Among the adolescent cases, 57 (73%) cases were female, 21(27%) cases were male, and a majority (80.8%) of them had completed 12 weeks of treatment.
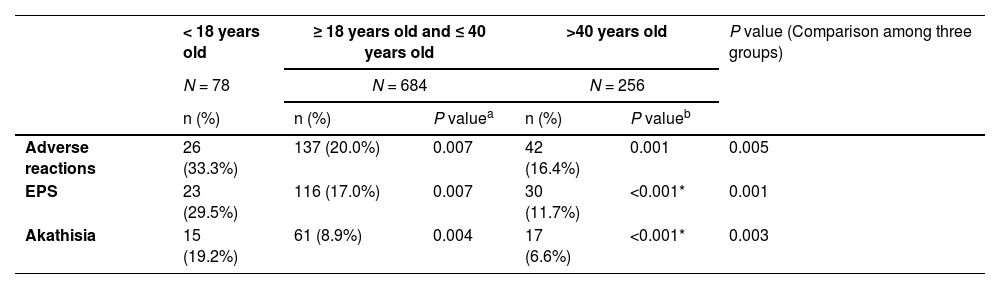
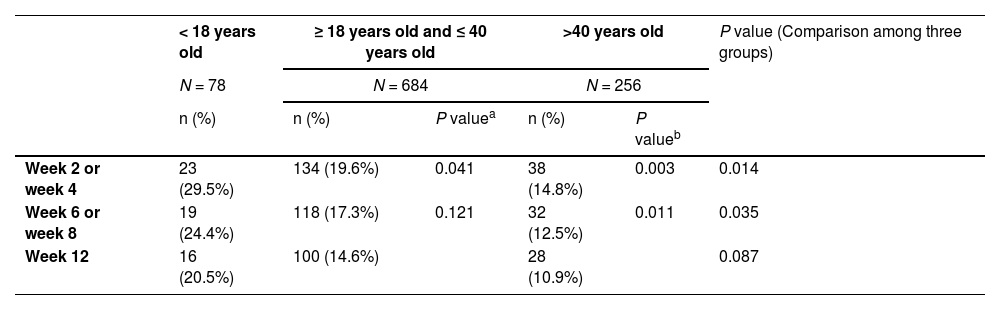
Safety analysisAs can be seen in Tables 1 and 2, adolescents were more likely to suffer from adverse drug reactions (ADRs), compared with adults, particularly the group that was older than 40 years old. Notably, adolescents had a significant higher percentage of extrapyramidal symptoms (EPS) combined treatment than other groups from week 2 to week 8. No significant difference was found at week 12, though the gap seemed remaining at that point.
ADRs occurring in different age.
| < 18 years old | ≥ 18 years old and ≤ 40 years old | >40 years old | P value (Comparison among three groups) | |||
|---|---|---|---|---|---|---|
| N = 78 | N = 684 | N = 256 | ||||
| n (%) | n (%) | P valuea | n (%) | P valueb | ||
| Adverse reactions | 26 (33.3%) | 137 (20.0%) | 0.007 | 42 (16.4%) | 0.001 | 0.005 |
| EPS | 23 (29.5%) | 116 (17.0%) | 0.007 | 30 (11.7%) | <0.001* | 0.001 |
| Akathisia | 15 (19.2%) | 61 (8.9%) | 0.004 | 17 (6.6%) | <0.001* | 0.003 |
Note:
The proportion of drugs for combined treatment of EPS in different ages.
| < 18 years old | ≥ 18 years old and ≤ 40 years old | >40 years old | P value (Comparison among three groups) | |||
|---|---|---|---|---|---|---|
| N = 78 | N = 684 | N = 256 | ||||
| n (%) | n (%) | P valuea | n (%) | P valueb | ||
| Week 2 or week 4 | 23 (29.5%) | 134 (19.6%) | 0.041 | 38 (14.8%) | 0.003 | 0.014 |
| Week 6 or week 8 | 19 (24.4%) | 118 (17.3%) | 0.121 | 32 (12.5%) | 0.011 | 0.035 |
| Week 12 | 16 (20.5%) | 100 (14.6%) | 28 (10.9%) | 0.087 | ||
Note:
Among the 78 adolescent patients included, 26 cases had adverse reactions, and the incidence of ADR was 33.3%; Among them, 23 patients developed EPS, and the incidence of EPS was 29.5%; 15 patients developed akathisia, and the incidence of akathisia was 19.2%. In addition, there were other adverse events such as abnormal liver function, and cardiovascular system, and nervous system abnormalities, but all of them were mild to moderate adverse events, and the incidence rate was less than 5%.
Weight analysisAn increase in weight is a common side-effect of psychotic drugs. Among 62 cases of adolescent patients, the researchers recorded an average weight change of 0.24±2.39 kg at the end of follow-up, and 8.1% of the patients experienced a weight gain equal to or larger than 7% in comparison with the baseline. Conclusively, patients under 18 years old did not exhibit a significant difference in weight gain, compared with other age groups. Paired t-test analysis of weight changes between each 2 groups showed no significant difference in the results either (Table 3).
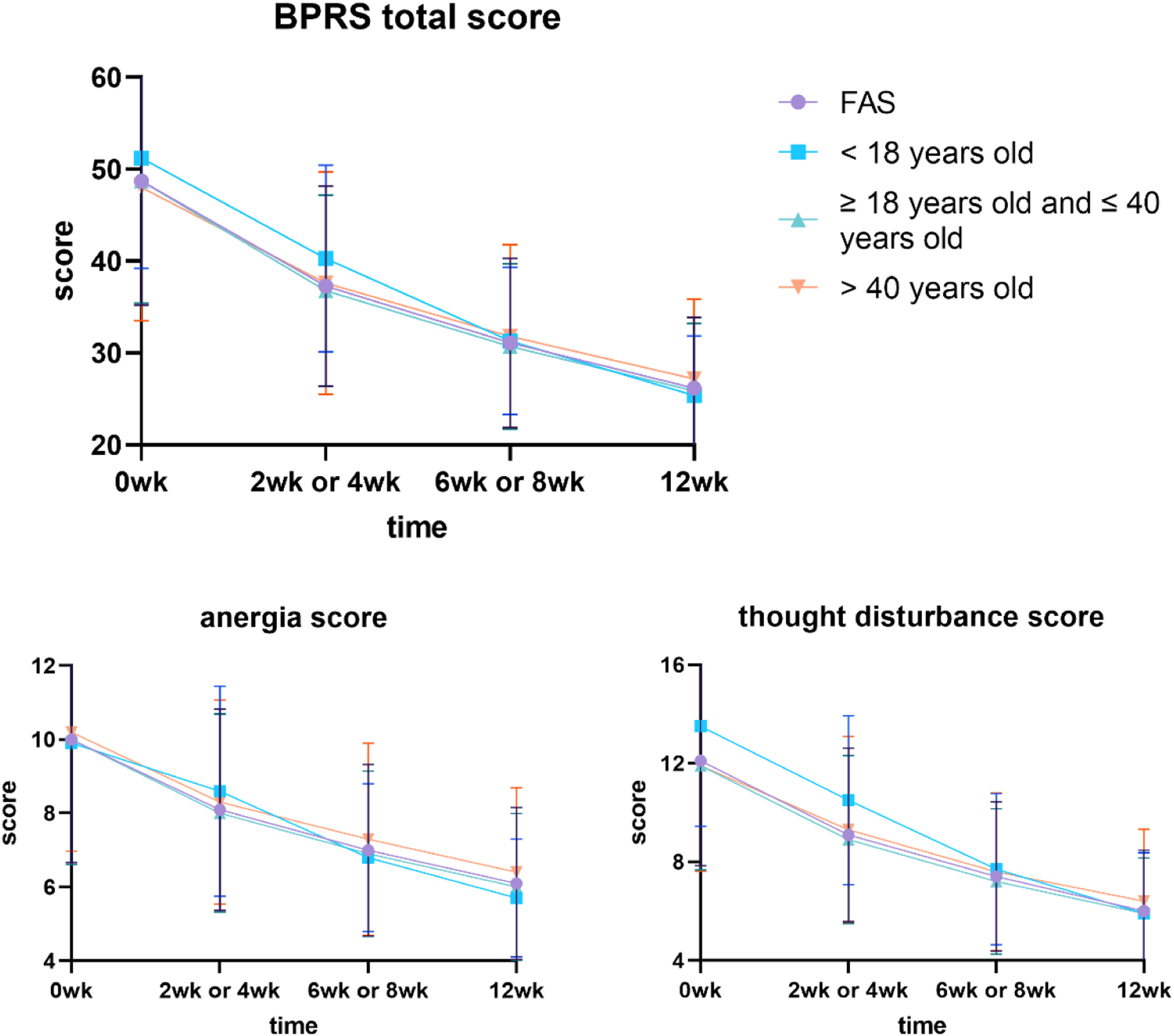
Effectiveness analysisThe total score of BPRS was significantly lower than the baseline (P < 0.001), which is similar to the changes observed in the overall population in the study. The mean decrease in BPRS total score was 48.7%±15.7% from the baseline to week 12. Approximately 43.6% (34/78) of adolescent patients achieved a ≥ 50% decrease in BPRS total score at week 12.
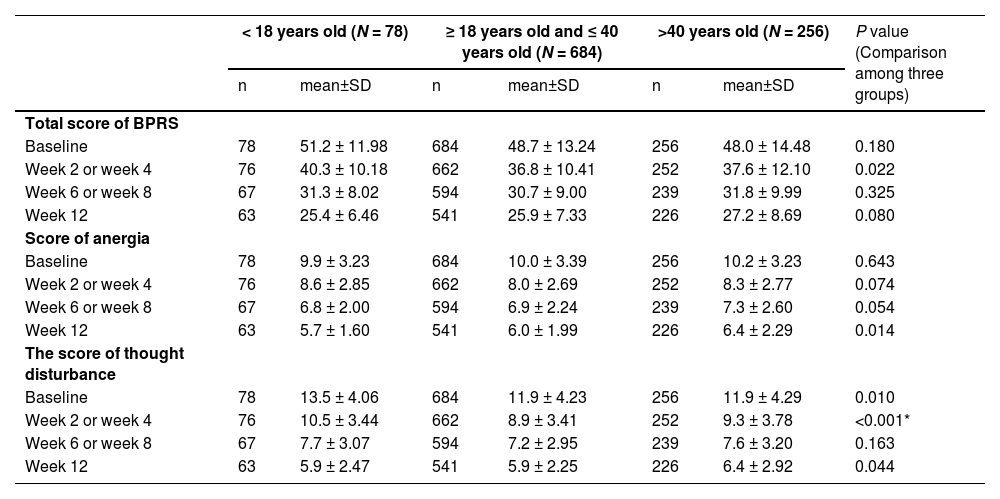
At week 2 or week 4, the p-values of the total BPRS score were 0.060 (< 18 vs. 18–40 years old) and 0.007 (< 18 vs.>40 years old). The p-values for < 18 vs. 18–40 years old group and < 18 vs.>40 years old group scores of anergia were 0.027 and 0.423 at 12 weeks. While the p-values for < 18 vs. 18–40 years old group and < 18 vs.>40 years old group scores of thought disturbance were 0.005 and 0.003 at week 0, 0.008 and < 0.001 at week 2 or week 4, and 0.204 and 0.906 at 12 weeks (Fig. 1, Table 4).
the total score and part of the subscale scores of BPRS in different ages.
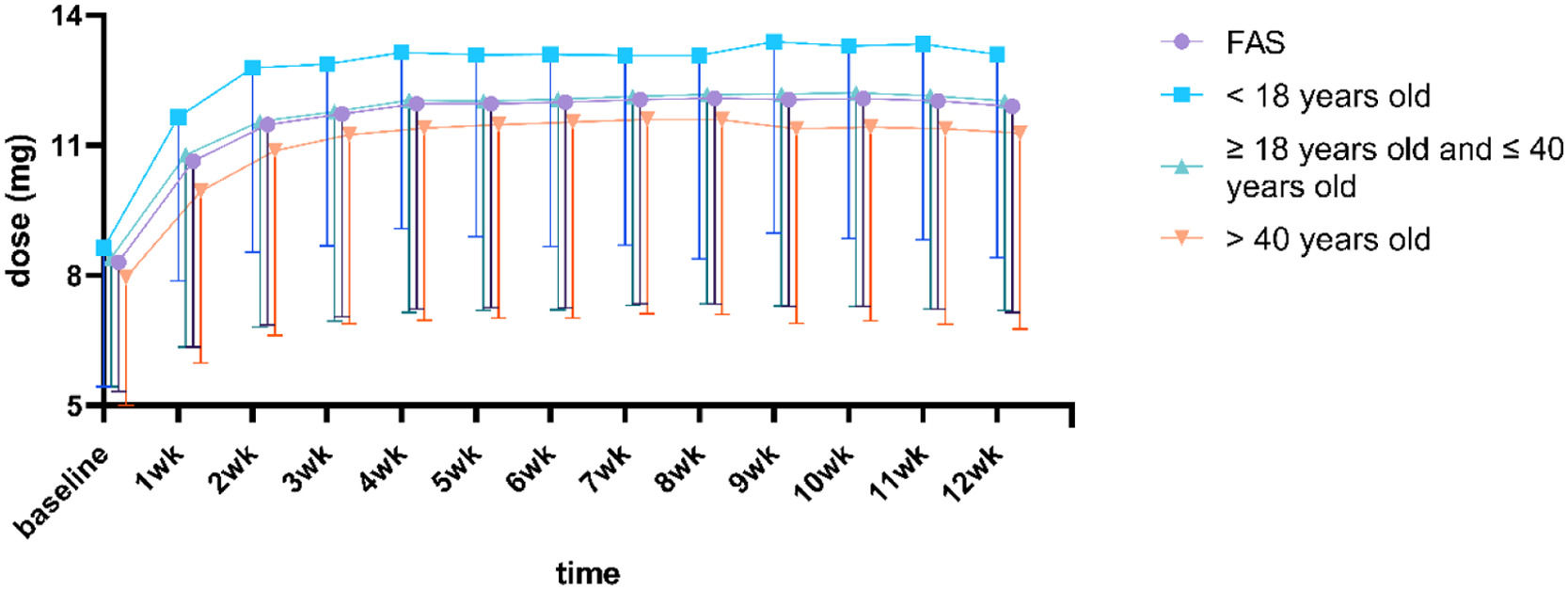
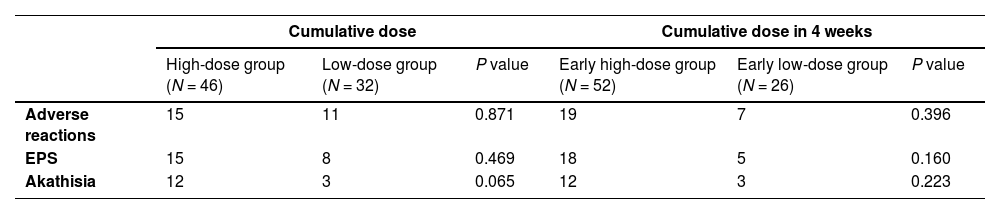
As shown by Fig. 2 and Table 5, the adolescent group had a higher blonanserin intake than other age groups at all stages of treatment. However, the current data did not suffice to support the correlation between cumulative dosage and adverse drug reactions toward blonanserin. The high-dose or low-dose group was defined as patients receiving ≥ or < 796 mg (the median cumulative dose) blonanserin during the entire treatment. The early high-dose group or early low-dose group was defined as patients receiving ≥ or < 260 mg (the median cumulative dose) blonanserin during the first 4 weeks of treatment.
DiscussionSafety of blonanserinAkathisia and extrapyramidal symptomsJapanese researchers had conducted PMS on blonanserin since its approval in 2008.8 The Japanese PMS recorded a 23.3% chance of ADR incident in 3130 cases, 4.3% for akathisia, and 2.4% for EPS (excluding akathisia).8 The results of our current study seem to be quite different from the PMS conducted in Japan. Dose differences, and different clinical diagnostic habits of adverse drug reactions are the hypothetical precursors of this contradiction in domestic PMS and Japanese PMS. In addition, the difference in the course of the disease may also lead to this result, because 21.8% of Japanese PMS have a course of ≥ 10 years, 34.6% have a course of ≥ 20 years, while the average course of disease in Chinese PMS is 63.8 months, and the course of disease in the adolescent population is theoretically even lower.
The domestic PMS research had a high percentage of blonanserin treatment continuation among the adolescents, as 80.8% of them completed the 12-week trial, a demonstration of exceptional tolerance of blonanserin for these patients. As a common ADR of psychotic drugs, EPS unsurprisingly topped the ADR record in research. The adolescent patients included in this analysis had a significantly higher incidence of EPS ADR, in comparison with the adult group. But the incidence of EPS was not significantly different from that of other second-generation antipsychotics like aripiprazole.9 According to Correll, et al., adolescents commonly bear a higher risk than other age groups for taking psychotic drugs.10 In this analysis, akathisia made up the largest portion of EPS. This result is consistent with the randomized controlled trial of blonanserin and risperidone in Chinese patients.11,12
Weight gainWeight gain is considered a vital factor associated with the clinical decision of second-generation antipsychotics, which could be extended to be a valuable indicator of medical compliance.13 Hence, psychiatrists should avoid prescribing any drugs that could cause weight gain in teenage patients.14 In this analysis, the proportion of patients with a weight increase ≥ 7% from baseline accounted for 8.1%. However, considering puberty, the real result is likely to be similar to some previous clinical studies,4,5,15 indicating that the risk of weight gain caused by blonanserin in clinical practice was low, which was also consistent with a Meta-analysis study in 2017.16 Moreover, considering that its statistical results are not significantly different from those of patients of other ages, the author concluded that blonanserin is moderately advantageous in the aspect of prevention of weight gain. The cause could be attributed to the receptor selectivity of blonanserin, as it has exceptionally low affinity to 5-HT2C, 5-HT1A, and H1 receptors, which are significant contributors to weight gain.17
Effectiveness of blonanserinIn terms of the Positive and Negative Syndrome Scale (PANSS) score, blonanserin had demonstrated an equivalent or even better performance than haloperidol and risperidone in numerous case-control research from the past.9,12,18 Several clinical studies have already shown that blonanserin was an efficient treatment for both positive and negative symptoms of schizophrenia, it could also improve the cognitive situation of the patients.19 Additionally, in studies that focused on adolescent schizophrenia patients, blonanserin exhibited considerable effect in improving the positive symptom, cognitive dysfunction, and negative symptoms.7 Notably, its effectiveness in relieving negative symptoms is very important in the treatment of adolescents, as negative symptoms are common in the early stage of schizophrenia, and tend to exert a lasting negative influence on the future development of the psychotic disorder. It could be the consequence of receptor selection and extraordinarily high affinity to dopamine D2, D3, and 5-HT2A receptors, with an average inhibition coefficient of 0.14, 0.49, and 0.81 nmol/L. Sakayori T, et al. conducted research on the clinical response by observing the receptor occupancy of blonanserin to dopamine D2 and D3 in thirteen patients with schizophrenia, suggesting its contribution to the improvement of cognitive impairments via D3 receptor antagonism.20
The research applied BPRS, an authentic evaluation model that is equivalent to PANSS, to evaluate the effectiveness of blonanserin, which is also often used in some previous studies of blonanserin.21 In every stage of the observation, the patient's BPRS score was exceptionally lower than the baseline; as the subscale score of BPRS, the anergia score, and the thought disturbance score was also significantly lower than the baseline; these results corresponded with the outcome of the previous studies.22,23 Negative schizophrenia symptoms are common in the first episode of schizophrenia and adversely affect the prognosis of subsequent diseases, which is especially obvious in adolescents. In the comparison between groups of different ages, we found that at the end of 12 weeks, the anergia score of adolescents was lower than that of the group of ≥ 18 years old and ≤ 40 years old, and the difference was significant. The thought disturbance score of adolescent patients was higher than that of other adult groups at baseline, which was also obvious in week 2 or week 4, and the difference was significant. However, at the end of 12 weeks, there was no significant difference between the adolescent group and the other groups. The assessment of the BRPS, anergia score is evaluated by the presence of multiple systems, including emotional withdrawal, motor retardation, blunted affect, and disorientation. Thought disturbance score is evaluated by conceptual disorganization, grandiosity, hallucination, and unusual thought content. A favorable score in these evaluations signals that blonanserin may be effective in relieving negative and positive schizophrenia symptoms, which is worthy of further study. Conclusively, blonanserin will be an ideal choice to provide effective treatment for adolescents who are suffering from schizophrenia.
Blonanserin dosingBased on the "Guidelines for the prevention and treatment of schizophrenia in China" and the previously mentioned results of PMS studies, the experts from China and Japan recommend an initial blonanserin dosage of 8 mg per day, divide in two times after a meal, and gradually increase the dosage to 12–24 mg per day within two weeks. For patients with acute schizophrenia, the experts recommend a higher dosage of prescription of 16–24 mg. As for the patients that receive maintenance treatment, the recommended dosage is 8–16 mg. Ultimately, all patients should not exceed the maximum dosage of 24 mg per day under any circumstance.24
In the third week of this study, the mean blonanserin intake for adolescents was 12.8 ± 4.3 mg per day, moderately higher than the dosage of the adult group with a mean intake of 11.6 ± 4.7 mg per day (18–40 years old) and 10.9 ± 4.3 mg per day (over 40 years). This difference between the adolescent group and adult group (over 40 years) existed in both the early (w. 2–4) and ending periods (w. 8–12) of the surveillance. The research team contended that the more energy expenditures of activity of adolescents might be the cause of this difference, but subsequent studies are needed to verify. Sub-population analysis was performed by dividing patients into two groups (high-dosage group and low-dosage group) based on the median cumulative drug dose of patients in the FAS. It was found that no correlation between cumulative intake of blonanserin and the incidence of ADR for adolescents. Based on these results, the author has reached the conclusion that blonanserin's metabolic risk is relatively negligible, and proceeds to suggest that adolescent patients begin the treatment with 8 mg per day, then gradually increase it to 12–16 mg per day.
LimitationFirst of all, the research in question did not contain control groups for crossed examination, nor any inclusion and exclusion criteria. Moreover, the essence of PMS research-non-interventional study-did not restrict the study objects’ usage of other drugs during the clinical trials, which would cause unwanted interferences inevitably. Lastly, the author suggests a combination of the BPRS score and PANSS score could produce a more authentic evaluation.
ConclusionOn the basis of the result of the referred studies, blonanserin exhibited favorable tolerance and safety, and a low risk of multiple side effects like metabolic dysfunctions and weight gain. Also, the favorable score of the BPRS evaluation in real-world clinical practice confirmed the effectiveness of blonanserin in schizophrenia treatments. Hence, the author has concluded that blonanserin is a reassuring choice for future schizophrenia treatment for adolescents due to its promising safety and effectiveness.
Ethical considerationsEthical approval was obtained from the ethics committees of the leading clinical site at the Second Xiangya Hospital of Central South University and the respective other study centers. Written informed consent from the subject's legally authorized representative was obtained or a waiver of informed consent was approved by the clinical site where the patients were enrolled.