The emergence of Covid-19 has affected all aspects of human life across the globe. Lockdowns everywhere are having dramatic social and economic consequences. No therapy has yet been approved, and vaccines are a priority potential tool to control the pandemic and its impacts. Multiple vaccines are in the last stage of the development process, but part of the population is not willing to get vaccinated for Covid-19. Several studies have examined the percentage of the population willing to get vaccinated, but few have analyzed the reasons for their decision. In this context, researching the factors influencing individuals’ intention to use a potential Covid-19 vaccine will be important to public health strategies. This paper analyzes these factors with an adapted Cognitive-Affective-Normative (CAN) model. Perceived vaccine efficacy is used as a cognitive variable, fear of the vaccine and fear of Covid-19 are used as affective variables, and social influence is used as the normative variable. The proposed model strongly explains the intention to use the Covid-19 vaccine (R2 = 0.81). The results show that vaccine efficacy will be the most important determinant of Covid-19 vaccine acceptance, followed by social influence. The findings can be very helpful for public health policies aimed at achieving widespread vaccination, a must for vaccine success.
Coronavirus disease 2019 (Covid-19) was first identified in December 2019 in Wuhan, China. Since then, the infection and death rates have been increasing around the world. According to the World Health Organization (WHO), the number of positive cases globally reached 32.7 million on September 27, 2020, with 991,000 deaths in 216 countries, areas, or territories (WHO, 2020). The pandemic has sent shockwaves through both society and the economy. Unemployment is on the rise, consumer spending is falling, and GDP in most countries is forecast to decline dramatically in the third and fourth quarters of 2020 (O'Grady, 2020). Most countries are expected to undergo recessions in 2020 (World Bank, 2020).
The only successful way to restore normality and start the economic recovery is to control the virus with effective treatments and vaccines. Until then, social distancing and lockdowns are the ways to control the pandemic.
No therapy has yet been approved by the W.H.O. or any country or regional organization, such as the US Food and Drug Administration (FDA) or its European equivalent, the European Medicines Agency (EMA). Given this state of affairs, vaccines seem like a potential solution to control the Covid-19 pandemic and its social and economic consequences. Even zero cases cannot guarantee the end of the pandemic if a therapy and/or vaccine is not discovered, tested, and approved. Accordingly, researchers around the world are working under pressure to find a Covid-19 treatment and vaccine in both cooperative and competitive processes carried out by private corporations and public agencies. Vaccines have become a geopolitical issue for countries such as China, Russia, and the US. The Covid-19 pandemic has reinforced the role of the state as a protector of society from outside threats (Heisbourg, 2020). The social and economic impact of an effective vaccine explains the global race to be the first to develop one. On August 12, 2020, Russia announced that it had registered the Sputnik V vaccine as the first vaccine against Covid-19. Other candidates from the US, UK, Germany, and China are in late-stage trials (Corera, 2020). But any medication, including vaccines, must follow a rigorous three-phase development process. In the first phase, the vaccine is tested on a small group of healthy people, to determine whether it is safe and if there are potential side effects. In the second phase, the vaccine's ability to induce immunity against the target virus is tested on a few hundred people, including a control group with a placebo. In the third phase, thousands of people are vaccinated to test the vaccine in the real world and determine whether it has any further side effects not discovered in the previous phases (CDC, 2014).
Because of the importance of vaccines to controlling the Covid-19 pandemic, the factors influencing acceptance of potential vaccines must be investigated. If a vaccine is ready but a large part of the population refuses to get it, it will not have beneficial effects. Several international studies have been conducted on the percentage of the population willing to get a Covid-19 vaccine. A recent study on its acceptance in the US found that 33% of the population would not accept it (Malik, McFadden, Elharake, & Omer, 2020). Another study shows that willingness to get vaccinated against Covid-19 is insufficient in 19 countries (Lazarus et al., 2020). The mere availability of a vaccine will not guarantee its success if a sufficiently high number of people do not get vaccinated. Researching the factors influencing willingness to get vaccinated is thus imperative. Vaccine acceptance could vary and be based on different factors (Dubé et al., 2018; Fu, Zimet, Latkin, & Joseph, 2017; Quinn, Jamison, An, Hancock, & Freimuth, 2019; Sarathchandra, Navin, Largent, & McCright, 2018). A search of the Scopus and WoS databases shows that no studies have been published in prestigious journals to date that use causal models with a high explanatory power regarding the intention to get vaccinated against Covid-19. This paper aims to help fill this gap by developing such a model for both Covid-19 vaccine acceptance and future research on the acceptance of other vaccines. Accordingly, it investigates the intention to use a potential Covid-19 vaccine using a modified version of the Cognitive-Affective-Normative (CAN) model (Pelegrín-Borondo, Reinares-Lara, Olarte-Pascual, & Garcia-Sierra, 2016), a model used to study the acceptance of innovative products and services (e.g. García-Milon, Juaneda-Ayensa, Olarte-Pascual, & Pelegrín-Borondo, 2020; Pelegrín-Borondo, Araújo-Vila, & Fraid-Brea, 2020; Reinares-Lara, Olarte-Pascual, & Pelegrín-Borondo, 2018). The proposed adaptation of the CAN model includes the impact of perceived vaccine efficacy, fear of the virus, fear of the vaccine, and social influence on the intention to use a potential Covid-19 vaccine. The findings will help health authorities promote Covid-19 vaccination among the population.
2Theoretical frameworkThe Cognitive-Affective-Normative model (CAN) was developed by Pelegrín-Borondo et al. (2016) to explain new product and service acceptance. The model was built based on the Theory of Reasoned Action (TRA) (Fishbein & Ajzen, 1975), the Theory of Planned Behavior (TBP) (Ajzen, 1991), the Technology Acceptance Model (TAM) (Venkatesh & Davis, 2000), and the Unified Theory of Acceptance and Use of Technology (UTAUT) models (Venkatesh, Davis, & Davis, 2003). The model defines three types of variables: cognitive (performance expectancy, effort expectancy), affective (positive emotions, negative emotions, anxiety), and normative (social influence). The model is well-suited to the present research goal, i.e., to analyze the factors influencing Covid-19 vaccine acceptance. The model proposed here is an adaptation of the CAN model tailored to this aim that uses vaccine efficacy as a cognitive variable, fear of the vaccine and fear of Covid-19 as affective variables, and social influence as the normative variable.
Vaccine efficacy refers to the percentage reduction in disease occurrence in vaccinated individuals compared to unvaccinated individuals under optimal conditions; it can be obtained through randomized clinical trials (Ainslie, Haber, & Orenstein, 2019). It is considered an important factor in motivating people to accept vaccination (Dubé et al., 2018). In fact, almost no vaccines are 100% efficacious, and not all people who get vaccines develop immunity against the target virus because of biological reasons that can differ from one person to another (WHO, 2018). The efficacy of a potential Covid-19 vaccine will impact acceptance, especially if the efficacy is low. In this context, the baseline vaccine efficacy seems likely to have a strong influence on the acceptance of a Covid-19 vaccine once it is brought to market (Harapan et al., 2020). For instance, if the Covid-19 vaccine is similar to the influenza vaccine, the expected efficacy could be equal to or lower than 55% (CDC, 2020). People prefer vaccines with high efficacy. Indeed, their willingness to accept vaccines could be negatively affected if their perception of the vaccine efficacy is low (Sun et al., 2020). For instance, less than 30% of US healthcare workers received the H1N1 vaccine, and less than 50% get the seasonal influenza vaccine each year due to their concerns regarding these vaccines’ efficacy (Poland, 2010). In light of these findings, the following hypothesis is proposed regarding the expected impact of vaccine efficacy on Covid-19 vaccine acceptance:
H1
People's intention to use the Covid-19 vaccine is positively affected by perceived vaccine efficacy.
In the CAN model, the affective dimension is formed by emotions. According to componential emotion theory, all emotions share certain common traits that define the concept of emotion (Pelegrín-Borondo, Arias-Oliva, & Olarte-Pascual, 2017; Russell, 2003, 2009). These traits are: an identifiable stimulus; a physiological reaction; a qualitatively unique feeling; a cognitive assessment; feelings of pleasure and/or displeasure; a tendency toward action; and the short-term nature of the process. In the academic literature, the basic emotions approach has been highlighted for the study of emotions. Under this approach, people are considered capable of recognizing their emotions and differentiating them from each other (Russell & Barrett, 1999). Thus, a series of basic emotions is proposed representing each category of emotion within the set of emotions a person can feel (Ekman, 1999; Ortony & Turner, 1990; Scherer, 2005). By way of comparison, emotions would be the basic colors in the infinite spectrum of existing colors. There are many lists of basic emotions. Fear is one of the basic emotions generally included in these lists (e.g., Izard, 1977 (DES scale); Richins, 1997 (CES scale); Watson, Clark, & Tellegen, 1988 (PANAS scale)). It has been widely shown to influence decisions about behavior (Russell, 2003) and demand for products (e.g., García-Milon et al., 2020; Han, Lerner, & Keltner, 2006; Jonathan & Mcgraw, 2009). In addition, emotions differ depending on the object triggering the emotion (Pelegrín-Borondo, Juaneda-Ayensa, González-Menorca, & González-Menorca, 2015). With vaccine acceptance, fear of the disease has been shown to produce different effects from fear of the vaccine (Anraad et al., 2020; Nguyen et al., 2020). In this sense, various studies have shown that fear of a disease positively affects acceptance of a vaccine to prevent it (Anraad et al., 2020; Nguyen et al., 2020; Patil, Patil, Ganla, & Durgawale, 2020). Likewise, fear of vaccine side effects has been shown to be an important and significant deterrent to deciding to get vaccinated (Abebe, Mengistu, & Mekuria, 2019; Anraad et al., 2020; Cordoba-Sanchez et al., 2019; Kyaw et al., 2019; Maltezou et al., 2019; Nguyen et al., 2020; Otieno et al., 2020), with fear of short-term and permanent side effects influencing behavior differently (Borena, Luckner-Hornischer, Katzgraber, & Holm-von Laer, 2016). In keeping with these findings, the following hypotheses regarding the impact of fear on vaccine acceptance are proposed:
H2
People's intention to use the Covid-19 vaccine is positively affected by fear of Covid-19.
H3
People's intention to use the Covid-19 vaccine is adversely affected by fear of the side effects of the Covid-19 vaccine.
Since individuals are members of social groups, other group members’ opinions and recommendations about a behavior or decision can influence them and guide that behavior or decision for them. These opinions and recommendations represent social influence, which refers to people's perception of performing a specific behavior based on the opinion of other people who are important to them (Venkatesh et al., 2003). The impact of social influence on behavioral intention has been established in the Theory of Reasoned Action (TRA) (Fishbein & Ajzen, 1975) and the Theory of Planned Behavior (TPB) (Ajzen, 1991). Previous research has established the importance of the opinion of those we consider important to vaccine acceptance (Abbas, Kang, Chen, Werre, & Marathe, 2018; Fu et al., 2017; Padhi & Almohaithef, 2020; Sarathchandra et al., 2018). In this context, recommendations from doctors and health institutions are expected to have a strong positive impact on people's intention to get a vaccine, especially in the case of Covid-19. This is because it is a new virus, and the only information available about it and any potential vaccines for it is provided by doctors, biologists, healthcare institutions, public authorities, and the scientific community. For instance, doctors’ recommendations to get a flu shot were found to have the greatest impact on vaccine acceptance (Harrison et al., 2018; Ramsey & Marczinski, 2011). In light of these findings, the following hypothesis is proposed:
H4
People's intention to use a Covid-19 vaccine is positively affected by a favorable social influence.
Based on the aforementioned hypotheses, the theoretical model shown in Fig. 1 is proposed.
3Methodology3.1Data collection, sample, and measuresTo test the proposed hypotheses, a survey was conducted of residents in Spain. Potential respondents were contacted by digital means and asked to participate. Gender quotas and three age groups were established. As the quotas for each age group were met, contact efforts were concentrated on the other groups. Although all the age group quotas were ultimately met, it was not possible to fully meet the gender quotas. The survey was self-administered and completed online. The information was collected from Tuesday, September 9, 2020, to Wednesday, September 16, 2020. A total of 600 valid surveys were obtained. Table 1 shows the data collection and sample characteristics.
Sample characteristics.
| Universe | Residents in Spain |
| Sample | 600 |
| Period | September 9, 2020 – September 16, 2020 |
| Gender | 45% Men; 55% Women |
| Age | 17 to 30 years old: 33%; 31 to 50 years old: 33%; 51 or older: 34% |
| Monthly income | Less than €1000: 6.3%; €1000 to €1749: 22.3%; €1750 to €2499: 20.0%; €2500 to €3000: 13.0%; More than €3000: 24.8%; No answer: 13.5% |
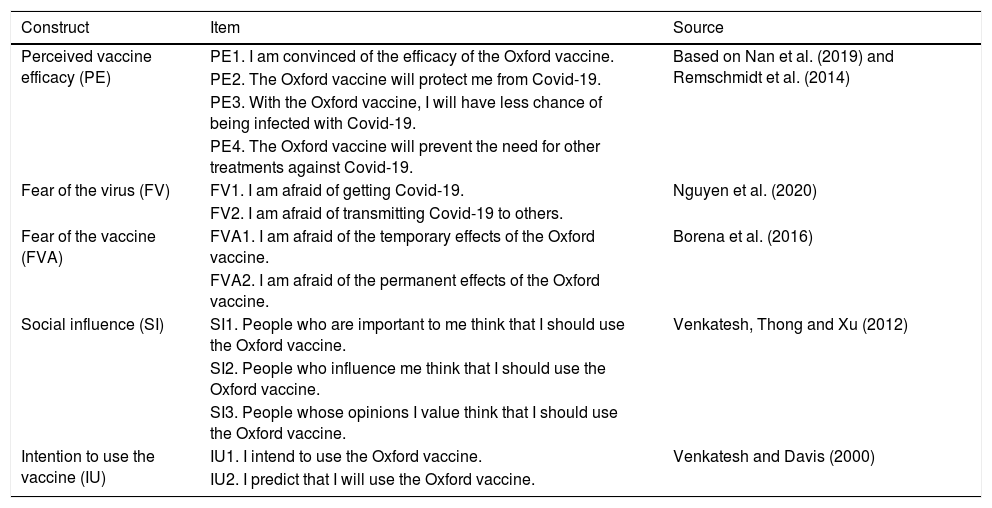
The survey questions were developed based on scales used by other researchers adapted for the present study. An 11-point Likert-type scale was used to score them, ranging from 0 (strongly disagree) to 10 (strongly agree). This type of scale is more sensitive and closer to normality than scales with a smaller range (Leung, 2011). Table 2 shows the variables used in the questionnaire.
Measurement scales.
| Construct | Item | Source |
|---|---|---|
| Perceived vaccine efficacy (PE) | PE1. I am convinced of the efficacy of the Oxford vaccine. | Based on Nan et al. (2019) and Remschmidt et al. (2014) |
| PE2. The Oxford vaccine will protect me from Covid-19. | ||
| PE3. With the Oxford vaccine, I will have less chance of being infected with Covid-19. | ||
| PE4. The Oxford vaccine will prevent the need for other treatments against Covid-19. | ||
| Fear of the virus (FV) | FV1. I am afraid of getting Covid-19. | Nguyen et al. (2020) |
| FV2. I am afraid of transmitting Covid-19 to others. | ||
| Fear of the vaccine (FVA) | FVA1. I am afraid of the temporary effects of the Oxford vaccine. | Borena et al. (2016) |
| FVA2. I am afraid of the permanent effects of the Oxford vaccine. | ||
| Social influence (SI) | SI1. People who are important to me think that I should use the Oxford vaccine. | Venkatesh, Thong and Xu (2012) |
| SI2. People who influence me think that I should use the Oxford vaccine. | ||
| SI3. People whose opinions I value think that I should use the Oxford vaccine. | ||
| Intention to use the vaccine (IU) | IU1. I intend to use the Oxford vaccine. | Venkatesh and Davis (2000) |
| IU2. I predict that I will use the Oxford vaccine. |
The adaptation of the questionnaire was influenced by several decisions:
- •
To facilitate interpretation by respondents, the questions were adapted to refer to the SARS-CoV-2 virus by its best-known name, namely, Covid-19.
- •
Because acceptance may vary between different vaccines, the questions focused on a particular vaccine. The validity of this assumption was confirmed by the survey results, as shown in the results section below. The selected vaccine was the one that, according to news reports, was the most advanced at the time (in accordance with the usual protocols for vaccine development) and had a high probability of being used in Spain, the country where the data were collected. Specifically, the vaccine being developed by the University of Oxford and the company AstraZeneca was selected.
- •
The decision to collect the information was made on the same day that news reports announced that the vaccine trials for the Oxford-AstraZeneca vaccine had been temporarily suspended due to a “serious adverse event” in a volunteer. In this sense, the questionnaire was prepared to account for this information as follows:
Suppose that the Covid-19 vaccine under development by the University of Oxford and AstraZeneca (“the Oxford vaccine”) is the first vaccine to be released on the market once the adverse effects have been addressed. The trials for this vaccine were suspended on September 9, 2020, after reports emerged of a “serious adverse event” in a volunteer. Please indicate the extent to which you agree or disagree with the following statements on a scale of 0 (strongly disagree) to 10 (strongly agree), where 5 is neither agree nor disagree.
Structural equation modeling (SEM) was used for the data analysis. Specifically, the Consistent Partial Least Square (PLSc) technique was used. This is because traditional Partial Least Squares (PLS) tends to skew factor loadings upwards and underestimate regression coefficients (Gefen, Rigdon, & Straub, 2011). Moreover, “PLSc avoids the excessive amount of Type I and Type II errors that can occur if traditional PLS or regression on sum scores is applied to estimate structural equation models with reflective measurement models” (Dijkstra & Henseler, 2015a, p. 299), as in the present case. PLS techniques were also chosen because they are less sensitive to the violation of data normality assumptions (Chin, 1998; Ram, Corkindale, & Wu, 2014).
4ResultsSince there are several vaccines for Covid-19 under development, the intention to use one in particular (the Oxford-AstraZeneca vaccine) to be vaccinated was analyzed. Among all the alternatives to this vaccine currently also under development, for the purposes of the present study, those furthest along in the development process were chosen (Sinovac Biotech, Sputnik V, and Moderna). Table 3 shows the mean value of the intention to use each of these vaccines and the standard deviations. Table 3 also shows the mean values and standard deviations of the variables used in the study.
Construct items, mean values, and standard deviations.
| Construct | Item | Mean value | Standard deviation |
|---|---|---|---|
| Intention to use vaccines | IU1. I intend to use the Sinovac Biotech vaccine (China). | 2.54 | 2.79 |
| IU2. I intend to use the Sputnik V vaccine (Russia). | 2.14 | 2.59 | |
| IU3. I intend to use the Moderna vaccine (US). | 3.89 | 3.04 | |
| IU4. I intend to use the Oxford-AstraZeneca vaccine. | 5.07 | 3.48 | |
| Perceived vaccine efficacy (PE) | PE1. I am convinced of the efficacy of the Oxford vaccine | 4.93 | 2.81 |
| PE2. The Oxford vaccine will protect me from Covid-19. | 5.31 | 2.80 | |
| PE3. With the Oxford vaccine, I will have less chance of being infected with Covid-19. | 5.95 | 2.97 | |
| PE4. The Oxford vaccine will prevent the need for other treatments against Covid-19. | 4.89 | 2.94 | |
| Fear of the virus (FV) | FV1. I am afraid of getting Covid-19. | 6.60 | 2.72 |
| FV2. I am afraid of transmitting Covid-19 to others. | 7.86 | 2.74 | |
| Fear of the vaccine (FVA) | FVA1. I am afraid of the temporary effects of the Oxford vaccine. | 6.75 | 3.02 |
| FVA2. I am afraid of the permanent effects of the Oxford vaccine. | 7.23 | 3.04 | |
| Social influence (SI) | SI1. People who are important to me think that I should use the Oxford vaccine. | 4.85 | 2.95 |
| SI2. People who influence me think that I should use the Oxford vaccine. | 4.64 | 2.95 | |
| SI3. People whose opinions I value think that I should use the Oxford vaccine. | 4.68 | 3.03 | |
| Intention to use the vaccine (IU) | IU1. I intend to use the Oxford vaccine. | 5.07 | 3.48 |
| IU2. I predict that I will use the Oxford vaccine. | 4.96 | 3.40 |
To verify the reliability of the scale items (Hair, Ringle, & Sarstedt, 2013), it was confirmed that all standardized loadings were greater than 0.7 and that the t-values were greater than 1.96 (see Table 4).
Standardized loadings (t-values), construct reliability, convergent validity, and discriminant validity.
| Construct/Item | Stand. loading (t-value) | CR | CA | AVE | PE | FV | FVA | SI | IU |
|---|---|---|---|---|---|---|---|---|---|
| Perceived vaccine efficacy (PE) | 0.94 | 0.93 | 0.79 | 0.89 | 0.49 | 0.28 | 0.72 | 0.86 | |
| PE1 | 0.95 (70.73) | ||||||||
| PE2 | 0.93 (81.72) | ||||||||
| PE3 | 0.90 (51.77) | ||||||||
| PE4 | 0.75 (25.84) | ||||||||
| Fear of the virus (FV) | 0.74 | 0.73 | 0.58 | 0.49 | 0.76 | 0.19 | 0.37 | 0.46 | |
| FV1 | 0.82 (22.48) | ||||||||
| FV2 | 0.71 (16.34) | ||||||||
| Fear of the vaccine (FVA) | 0.92 | 0.92 | 0.86 | −0.29 | 0.19 | 0.93 | 0.30 | 0.35 | |
| FVA1 | 0.96 (34.59) | ||||||||
| FVA2 | 0.89 (32.43) | ||||||||
| Social influence (SI) | 0.97 | 0.97 | 0.92 | 0.72 | 0.37 | −0.30 | 0.96 | 0.79 | |
| SI1 | 0.96(84.78) | ||||||||
| SI2 | 0.98 (85.20) | ||||||||
| SI3 | 0.94 (61.61) | ||||||||
| Intention to use the vaccine (IU) | 0.95 | 0.95 | 0.90 | 0.86 | 0.45 | −0.35 | 0.79 | 0.95 | |
| IU1 | 0.97 (131.8) | ||||||||
| IU2 | 0.93 (81.93) | ||||||||
Notes: Stand. loading = standardized loading; CR = composite reliability; CA = Cronbach's alpha. The diagonal elements (in bold) are the square root of the AVEs. The off-diagonal elements are the inter-construct correlations. The elements above the diagonal are the HTMT values.
To verify construct reliability, Cronbach's alpha and the composite reliability of the constructs were analyzed. Table 4 shows that the reliability was adequate: Cronbach's alpha and composite reliability returned values greater than 0.7. The average variance extracted (AVE) was greater than 0.5, confirming the convergent validity of all constructs. The discriminant validity criterion was also met (Roldán & Sánchez-Franco, 2012): the square root of each construct's AVE was greater than the inter-construct correlations, and the HTMT was less than 0.9 in all cases.
The collinearity between the antecedents of the intention to use (i.e., perceived vaccine efficacy, fear of the virus, fear of the vaccine, and social influence) was also assessed. The highest variation inflation factor (VIF) was 2.48 for perceived vaccine efficacy. There may be multicollinearity problems if the VIF is higher than 10 (Petter, Straub, & Rai, 2007).
4.2Assessment of the structural modelTable 5 and Fig. 2 show the effects of the exogenous variables on the intention to use the Oxford-AstraZeneca vaccine. The proposed model worked quite satisfactorily. The coefficient of determination (R2) was 0.812. This result indicates that 81.2% of the variance in the intention to use the vaccine is explained by the model's exogenous variables. According to Chin (1998), R2 values of 0.67 or greater can be considered substantial. Therefore, the present model more than substantially explains the variation in the intention to use the Oxford-AstraZeneca vaccine. The model's predictive power was analyzed using the Q2 provided by PLS Predict. In this case, the Q2 had a value of 0.744. As the Q2 was greater than 0, the exogenous variables do predict the endogenous variable (Shmueli, Ray, Velasquez-Estrada, & Chatla, 2016). The SRMR provided by the program SmartPLS3 has been of 0.02 and NFI has been 0.97.
Effect on the endogenous variable.
| R2 | Q2 | Direct effect | p-value | Correlation | Variance explained | |
|---|---|---|---|---|---|---|
| Intention to use | 0.812 | 0.744 | ||||
| H1: Perceived vaccine efficacy (PE)=> (+) Intention to use | 0.57 | < 0.00 | 0.86 | 49.02% | ||
| H2: Fear of the virus (FV) => (+) Intention to use | 0.08 | < 0.02 | 0.45 | 3.60% | ||
| H3: Fear of the vaccine (FVA) => (+) Intention to use | −0.11 | <0.00 | −0.35 | 3.85% | ||
| H4: Social influence (SI) => (+) Intention to use | 0.32 | < 0.00 | 0.79 | 25.28% |
Support was found for all the proposed hypotheses (H1, H2, H3, and H4). Perceived vaccine efficacy (H1) accounted for the highest percentage of variance explained in the intention to use the Oxford-AstraZeneca vaccine (49.02%), followed by social influence (25.28%, H4), fear of the vaccine (3.85%, H3), and fear of the virus (3.60%, H2).
5Discussion and implicationsAs expected, the impact of perceived vaccine efficacy on Covid-19 vaccine acceptance was confirmed, and it explained the highest percentage of variance in the intention to use the Oxford-AstraZeneca vaccine. This result is similar to those of previous studies on vaccine acceptance, where high perceived vaccine efficacy is considered one of the main drivers of vaccine acceptance (Alkuwari, Aziz, Nazzal, & Al-Nuaimi, 2011; Nguyen et al., 2020; Oldin, Golsäter, Schollin Ask, Fredriksson, & Stenmarker, 2019; Teo, Smith, Lwin & Tang, 2019). Accordingly, lower perceived efficacy could lead to the vaccine's rejection (Abbas et al., 2018; Dubé et al., 2018; Salmon et al., 2005). The vaccine efficacy test is performed in Phase 3 of the vaccine development process, in which the scientists give the vaccine to thousands of volunteers and see how many individuals are infected. The trials can thus determine whether or not a potential vaccine will protect against the virus (Grady et al., 2020). According to the New York Times Coronavirus Vaccine Tracker, nine vaccines are currently in Phase 3 efficacy trials. The four Covid-19 vaccines cited in this research are all in Phase 3. The Sinovac Biotech (China) and Sputnik V (Russia) vaccines have been approved for limited use, the Moderna vaccine company (US) lost a patent dispute over some of the technologies being used to develop the vaccine, and the Oxford-AstraZeneca vaccine is in Phase 2/3 of the trials (Corum, Wee, & Zimmer, 2020). In addition to its importance to determining the safety and efficacy of the vaccine, the outcome of this phase will be the basis for the formulation of its perceived efficacy among the population. Positive outcomes will make it easier to convince individuals about the potential vaccine's efficacy and, thus, increase acceptance of it (Esen & Derya, 2010).
Social influence had a highly significant impact on Covid-19 vaccine acceptance, explaining the second-highest percentage of variance in the intention to use the Oxford-AstraZeneca vaccine. This impact has been confirmed in previous vaccine acceptance studies (Abbas et al., 2018; Fu et al., 2017; Padhi & Almohaithef, 2020; Sarathchandra et al., 2018). The general population's understanding and knowledge of the Covid-19 virus is still low. For instance, there is still uncertainty regarding the virus's origin, its symptoms, how long immunity lasts, and whether the virus will evolve to be less deadly or will mutate, rendering the vaccines ineffective; this is in addition to the conflicting news about the virus and its vaccine (Gallagher, 2020). This uncertainty could confuse people and increase their reliance on recommendations from others, such as family members, biologists, doctors, or governments (Sarathchandra et al., 2018). In this regard, one study has found that countries with higher levels of trust in information from government sources are more likely to accept a vaccine (Lazarus et al., 2020). The present findings are consistent with such results, finding the social norm to be a very influential factor in vaccine acceptance.
The impact of fear of the vaccine and fear of Covid-19 has also been confirmed, with fear of the vaccine having a significant negative impact on the intention to use the Oxford-AstraZeneca vaccine and fear of Covid-19 positively impacting the intention to use it. These results are similar to those of previous research on the impact on vaccine acceptance of fear of a vaccine (e.g., Abebe et al., 2019; Anraad et al., 2020; Cordoba-Sanchez et al., 2019; Kyaw et al., 2019; Maltezou et al., 2019; Nguyen et al., 2020; Otieno et al., 2020) and fear of a viral infection (e.g., Anraad et al., 2020; Nguyen et al., 2020; Patil et al., 2020). The broad impact of the Covid-19 pandemic on all aspects of human life, including financial, social, and health aspects, could push people to accept a potential vaccine to control the pandemic. Most previous research on vaccine acceptance has found that fear of a viral infection has the greatest impact on vaccine acceptance. However, the present results show that fear of the virus has the smallest impact on the intention to use the Oxford-AstraZeneca vaccine. This may be due to the point we have reached in the pandemic, where vaccine development is in the final stages and we have been living with the pandemic for a long time, with all the negative impacts on all aspects of human life that entails. The expectations that it could be with us for some time still may redirect people's attention to controlling the pandemic rather than to the virus itself. Additionally, throughout the pandemic, public sentiment has shifted from fear to anger (Lwin et al., 2020).
Finally, the present results indicate that people prefer the Oxford-AstraZeneca vaccine to the Moderna vaccine (US), Sinovac Biotech vaccine (China), and Sputnik V vaccine (Russia), in that order. These preferences could be a result of the influence of the social norm. The current geopolitical situation between the US, Russia, and China could explain it. In this context, media narratives about the potential vaccines heighten the differences in the perception of each one. For instance, researchers at US government institutions have expressed concern about political interference to speed up the vaccine development process, which could affect the safety and effectiveness of potential vaccines (LaFraniere, Thomas, Weiland, Baker, & Karni, 2020). There are likewise doubts about the credibility of the Chinese and Russian vaccines because of the opacity of their development processes, with some Western countries even accusing China of causing the current pandemic and concealing information about Covid-19 in its early stages. Similarly, there are concerns about the Russian vaccine's safety and efficacy, because the vaccine was registered before completing the Phase 3 trial (Corera, 2020).
The present findings suggest that public policy should focus on vaccine efficacy and communication strategies involving influential people in order to increase the percentage of people willing to be vaccinated. It is recommended to analyze the capacity of influence of different social entities: doctors with direct contact with people, government sources, politicians, journalists, etc. The information about the vaccine's efficacy and recommendations on vaccination should be transmitted by the most influential ones. Messages intended to stoke fear about the effects of the virus and concerning the side effects of vaccines will not be as critical in fostering acceptance.
6Limitations and further researchCovid-19 has a longer median incubation period and longer serial interval than, for instance, influenza. It can moreover be spread quickly and easily to a lot of people, and it is more infectious for certain age groups and populations than flu (CDC, 2020). In addition to the global lockdown and the huge impact the pandemic has had on all aspects of our lives, these facts could make the factors influencing Covid-19 vaccine acceptance and their respective intensities different from those affecting acceptance of other vaccines. Additional research should thus study differences between acceptance of other vaccines and acceptance of the Covid-19 vaccine, once it becomes available on the market. This result also points to differences in the intention to use different vaccines. Results of this research focus on the Oxford and AstraZeneca vaccine. It would be advisable to replicate the research for other vaccines for Covid-19 and check whether the results are similar. Future research should thus study the impact of the geopolitical situation, conflicting media narratives, and country image on vaccine acceptance as well. The present study was conducted in a single country. It should be extended to other countries and regions, too, in order to understand cultural differences and their impact on Covid-19 vaccine acceptance. Specific studies, such as a comparison between regions with higher and lower infection rates, could be very useful for public policy. The model does not include other variables showed in literature that could influence the acceptance of vaccines, in particular the perceived risk. Perceived risk of the vaccine and the disease were not included because the risk produces fear, and fear of disease and of vaccine are variables incorporated in the model. A quite surprising result is that social influence showed a much greater influence on vaccine acceptation than fear. A further analysis about social influence and fear is recommended. This research has not taken into account the indirect effects of social influence on the intention to use the vaccine. Future studies could address how social influence affects vaccine acceptance through efficacy or fear.
This research has been funded by the Autonomous Community of La Rioja (Spain) and the University of La Rioja through the program Joint programming actions (Reference: CAR -PID2019-105764RB-I00).