Biliary complications occur in 5–25% of patients after liver transplantation and represent a major source of morbidity in this group of individuals. The major risk factor for most of these complications is ischemia of the bile tree usually due to obstruction or vascular insufficiency of the hepatic artery. The most common complications include biliary strictures (anastomostic and nonanastomotic), bile leaks, and biliary filling defects. The initial diagnostic approach starts with a high index of suspicion along with an abdominal ultrasound and Doppler exam. Magnetic resonance imaging is highly sensitive and is usually reserved for confirmation. The vast majority of these complications can be successfully treated with endoscopic retrograde cholangiography, however if this procedure cannot be performed a percutaneous approach or surgery is recommended. Nonanastomotic strictures and living donor recipients present a less favorable response to endoscopic management. This review focuses on the current diagnostic and therapeutic approaches for the management of biliary complications after liver transplantation.
Entre el 5 y el 25 por ciento de los pacientes sometidos a trasplante hepático sufren complicaciones biliares. Éstas representan una causa importante de morbilidad en este grupo. En la mayoría de los casos, el principal factor de riesgo es la isquemia del árbol biliar, normalmente debida a la obstrucción o a insuficiencia vascular de la arteria hepática. Las complicaciones más comunes son la estenosis biliar (anastomótica y no anastomótica), fuga biliar y litiasis biliar. El enfoque de diagnóstico inicial se basa en un alto índice de sospecha al que le sigue un examen ecográfico del abdomen. La resonancia magnética es muy sensible y se reserva para la confirmación. La gran mayoría de estas complicaciones puede tratarse con éxito mediante colangiografía retrógrada endoscópica, pero si este procedimiento no es posible, se recomienda un enfoque percutáneo o quirúrgico. La estenosis no anastomótica y los receptores de hígados procedentes de donantes vivos presentan una respuesta menos favorable ante el manejo endoscópico. Esta revisión se centra en los enfoques terapéuticos actuales para el manejo de complicaciones biliares asociadas al trasplante hepático.
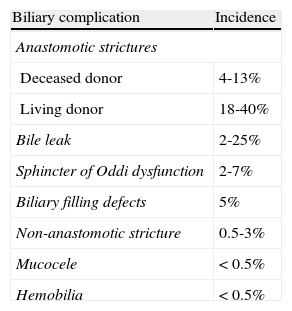
Biliary tract complications can occur in 5–25% of patients following liver transplantation (LT).1–4 The most frequent complications are biliary strictures, anastomotic leaks, and biliary filling defects. Other complications include biloma, sphincter of Oddi dysfunction, mucocele, and hemobilia (Table 1). The development of these complications is related to several risk factors that ultimately lead to fibrosis and stenosis of the bile duct, wound dehiscence of the anastomosis and/or stone and sludge formation in the bile duct. The most common risk factors implicated in the development of biliary complications after LT are related to: (1) graft-related factors such as donation after cardiac death, older age of donor, ABO mismatch and prolonged cold and warm ischemia time,5,6 (2) peri-operative factors, mainly hepatic artery complications, technical factors during surgery, T-tube placement, and presence of bile leak,6–10 and (3) nonoperative factors that include cytomegalovirus infection and a previous diagnosis of primary sclerosing cholangitis.11
Incidence of biliary complications after liver transplantation
| Biliary complication | Incidence |
| Anastomotic strictures | |
| Deceased donor | 4-13% |
| Living donor | 18-40% |
| Bile leak | 2-25% |
| Sphincter of Oddi dysfunction | 2-7% |
| Biliary filling defects | 5% |
| Non-anastomotic stricture | 0.5-3% |
| Mucocele | < 0.5% |
| Hemobilia | < 0.5% |
The type of liver transplantation and biliary reconstruction has some implications in the development of biliary complications. Due to the small diameter of the anastomotic bile duct, biliary strictures are known to be more common in living-donor LT (LDLT) than in deceased-donor LT (DDLT).12–15 The type of biliary reconstruction (duct-to-duct choledocho-choledochostomy versus Roux-en-Y choledochojejunostomy) in DDLT has been suggested as a risk factor for biliary complications, however it is now generally agreed upon that the rate of complications is similar with the Roux-en-Y choledochojejunostomy. In most centers duct-to-duct anastomosis is preferred as it offers the advantage of easy endoscopic access to the biliary system and preservation of the sphincter of Oddi which might reduce the risk of cholangitis and stones.16 However, in LDLT with small-sized ducts (<4mm in diameter) hepatico-jejunostomy anastomosis presents a lower percentage of biliary complications compared to duct-to-duct anastomosis.17
A multidisciplinary team approach including hepatologists, endoscopists, transplant surgeons, and radiologists is strongly recommended for both the diagnosis and management of these patients. This article will review the current diagnosis and management of biliary complications after LT.
Diagnostic approachThe clinical presentation of biliary complications is variable according to the type of lesion. In many cases patients will have nonspecific symptoms such as malaise and anorexia. Others may present with mild abdominal discomfort, pruritus, jaundice, or bile ascites. A biliary complication usually is first suspected in asymptomatic LT recipients who have elevations of serum bilirubin, alkaline phosphatase and/or gamma-glutamyl transferase levels. Patients with bile leaks may present with acute abdominal pain in the perioperative period or after the removal of T-tube. Strictures usually present as asymptomatic cholestasis although some patients can present cholangitis especially if the patient has concomitant bile duct stones.
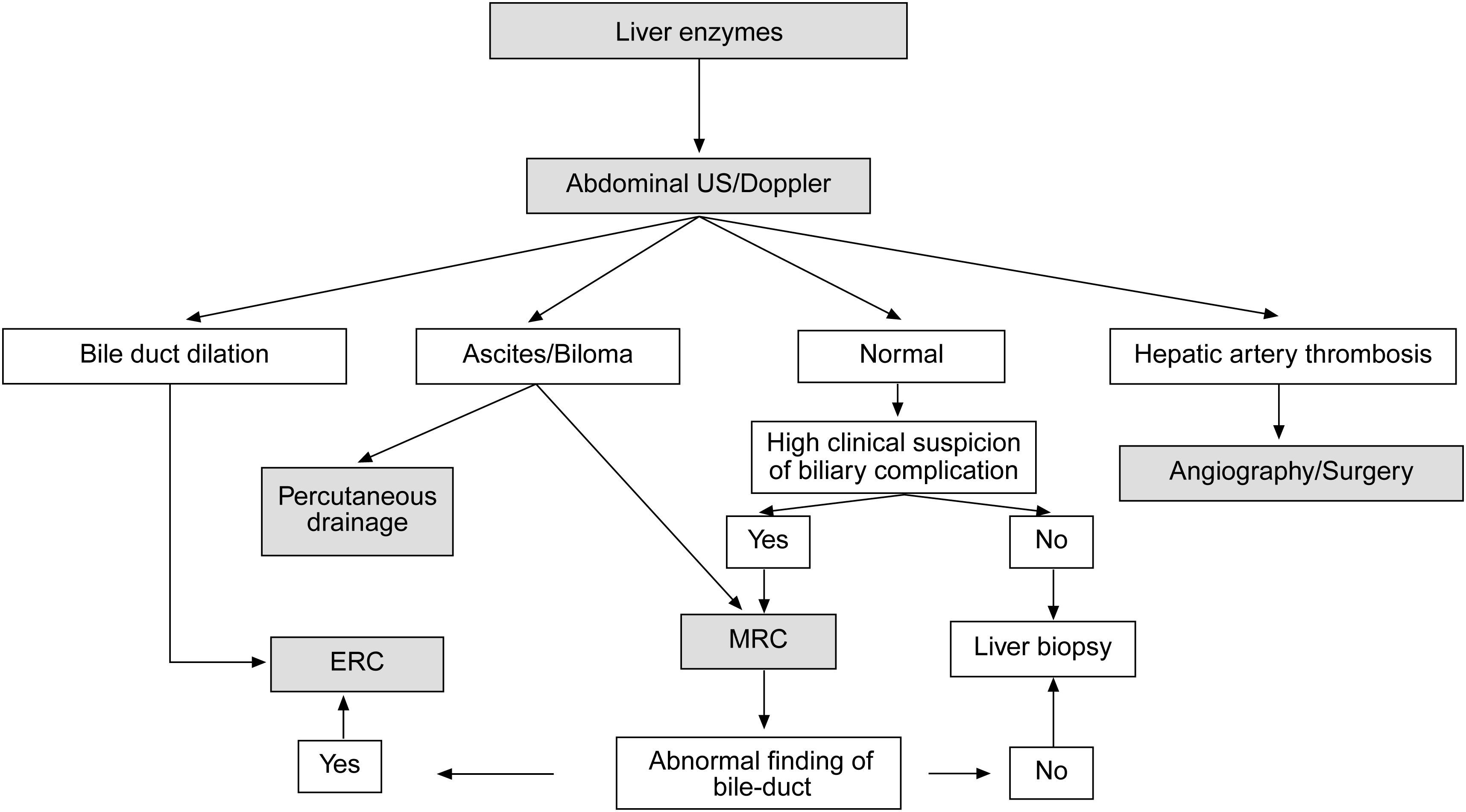
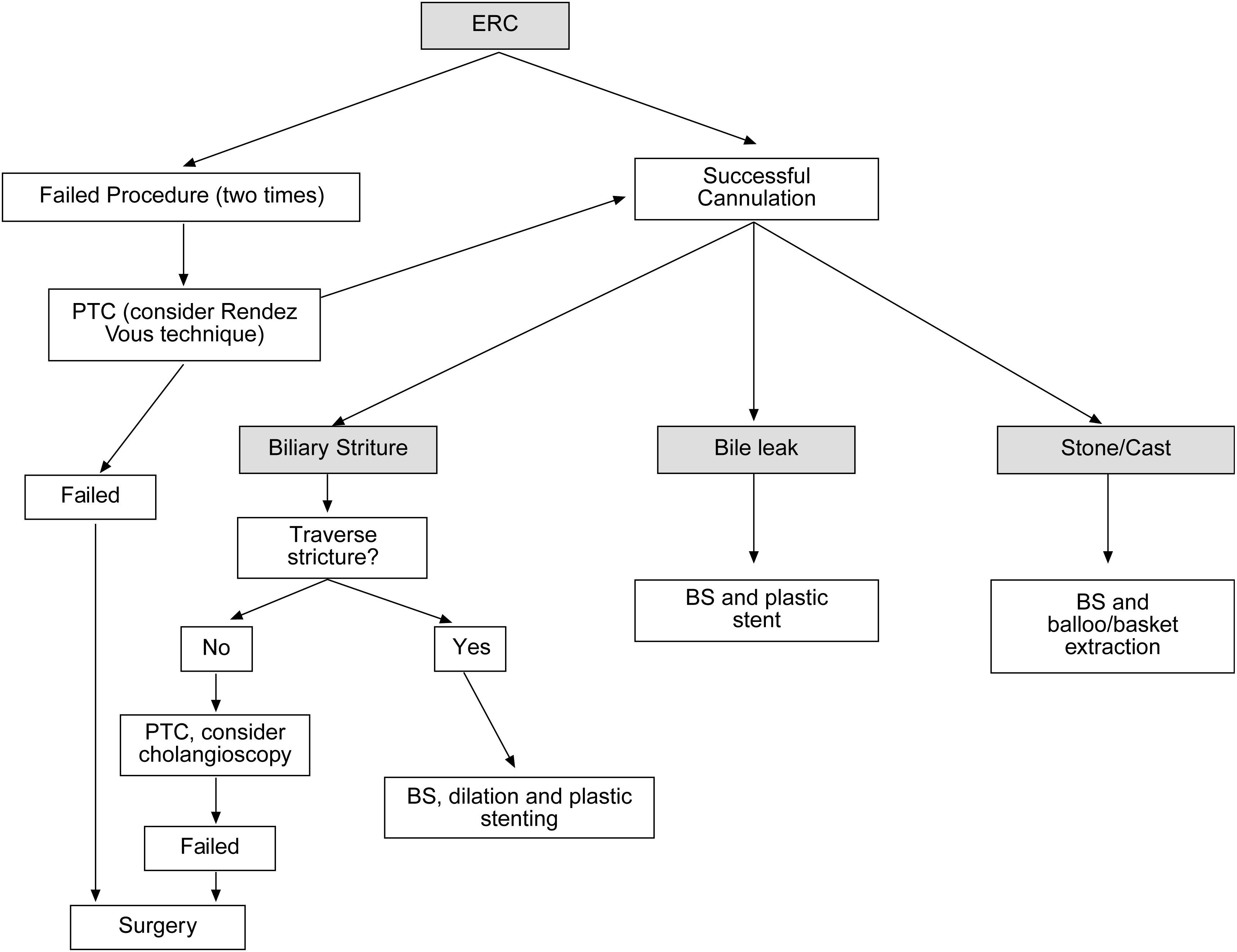
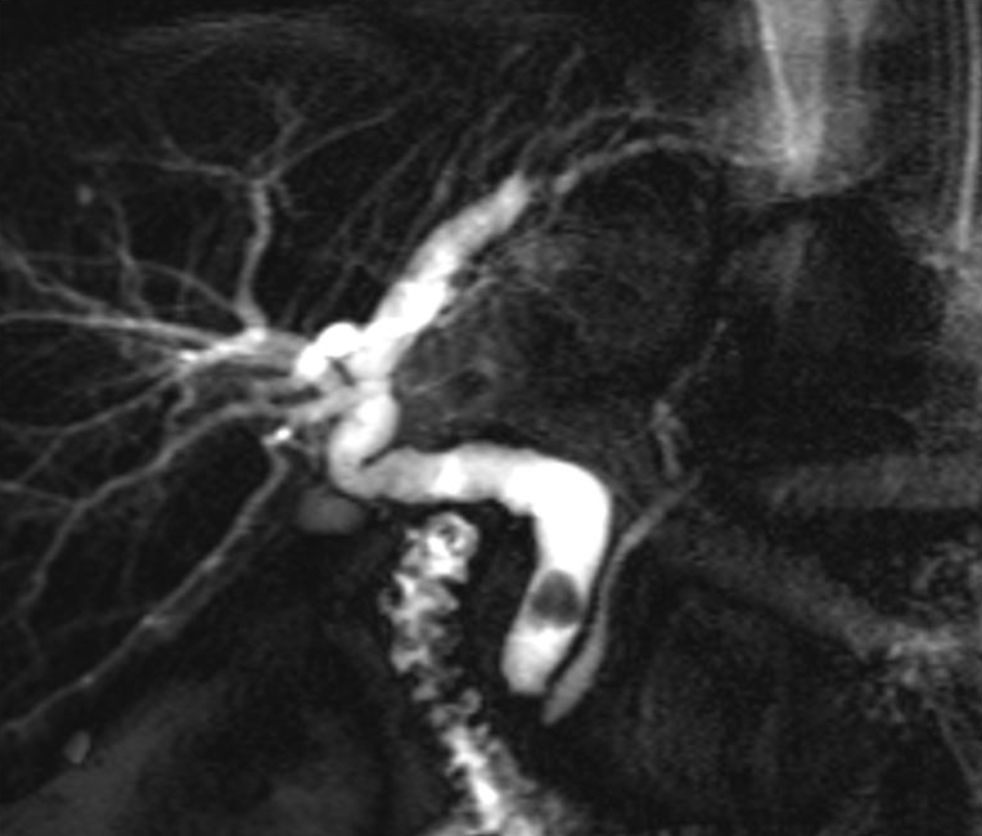
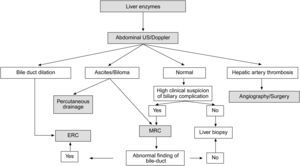
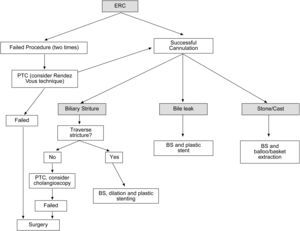
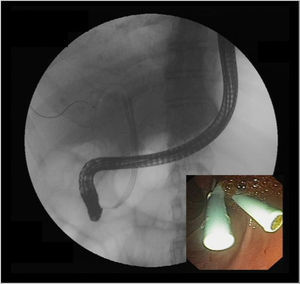
When a biliary complication is suspected, the initial evaluation should include liver blood tests and an abdominal ultrasound (US) with a Doppler evaluation of the hepatic vessels (Figure 1). It is important to take into account that an abdominal US may not be sufficiently sensitive (sensitivity 40–70%) to detect biliary dilation in the post-LT setting and a normal US should not preclude further evaluation with more sensitive techniques in patients in whom there is a high clinical suspicion of biliary tract complications.18 If the clinical suspicion is high and the ultrasound is normal, a magnetic resonance cholangiopancreatography (MRCP) is the next step. MRCP has a good accuracy for detecting biliary complications after LT (sensitivity of 93–98% and a specificity of 92–98%) compared with endoscopic retrograde cholangiography (ERC) as the standard reference.19–22 Furthermore, MRCP can provide the endoscopist or interventional radiologist with a map of the biliary tree and the localization of specific lesions, particularly those in the hiliar or proximal intrahepatic regions. However, an MRCP has a limited ability to detect biliary sludge, small stones, and ampullary lesions. Patients with high suspicion of a biliary complication and negative results of the abdominal US or MRCP should undergo a liver biopsy to exclude rejection or other causes of cholestasis. If the liver biopsy excludes other causes of cholestasis and detects bile duct proliferation indicative of biliary obstruction, an invasive approach should be performed. ERC is the preferred initial test in these patients as it helps confirm the diagnosis and allows therapy (Figure 2). A percutaneous transhepatic cholangiography (PTC) generally should be reserved for patients in whom ERC was unsuccessful or in patients with a Roux-en-Y choledochojejunostomy. In centers with experience, small bowel balloon enteroscopy ERC is used previously to PTC in patients with a Roux-en-Y anastomosis.23,24
Suggested algorithm for liver transplant recipients that present with abnormal liver enzymes and a suspected biliary complication after liver transplantation. If the patient has a T-tube in place a diagnostic cholangiogram can be performed in those with a high clinical suspeicion of biliary obstruction or bile leak. MRC: magnetic resonance cholangiography, ERC: endoscopic retrograde cholangiography.
Bile duct strictures are the most common biliary complication after LT and account for approximately 40% of all biliary complications.1–5,15,25 They occur in 4–13% of patients after deceased-donor LT and in 18–40% of patients after living donor LT.1–5,15,25
Strictures can be categorized as early (within the first month of LT) or late (1 month after LT).26 Strictures that occur early after LT are due mostly to technical problems, whereas late strictures are mainly due to vascular insufficiency and problems with healing and fibrosis.16,27 Furthermore, bile leak is an independent risk factor for the development of early or late strictures and that is why a bile leak requires urgent therapy.6 This is also important because the median duration of endoscopic therapy to reach initial success is much longer (up to 2 years) in patients with late-onset strictures.3,26 According to the localization, strictures are classified as anastomotic (AS) or nonanastomotic (NAS). NAS occur more than 0.5cm proximal to the biliary anastomosis. The two types of strictures differ in presentation, response to therapy, and outcome.
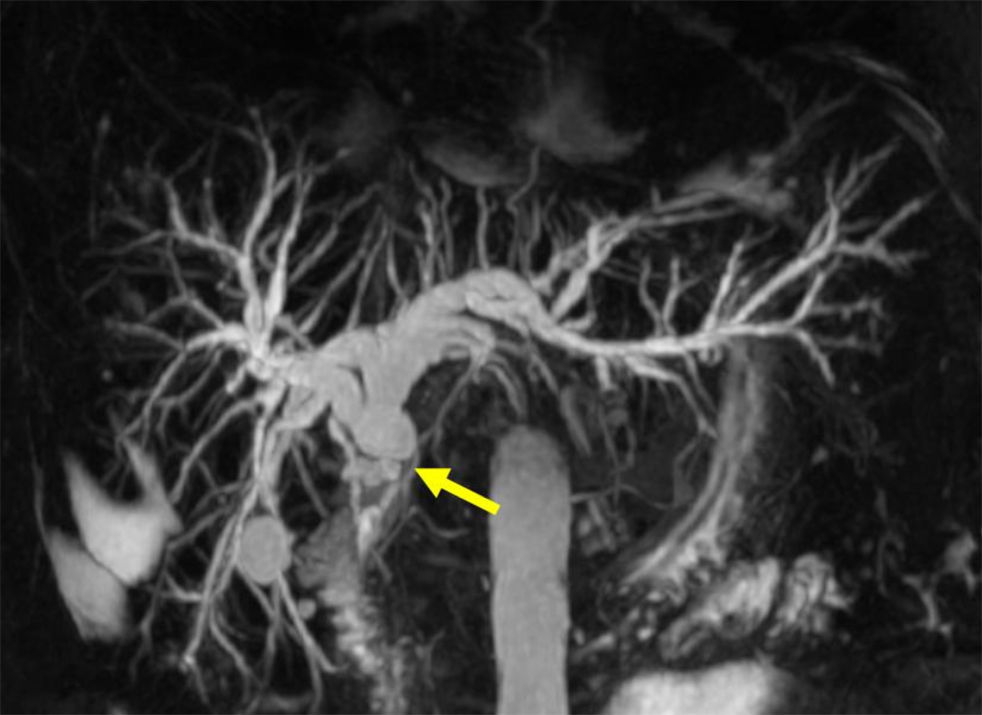
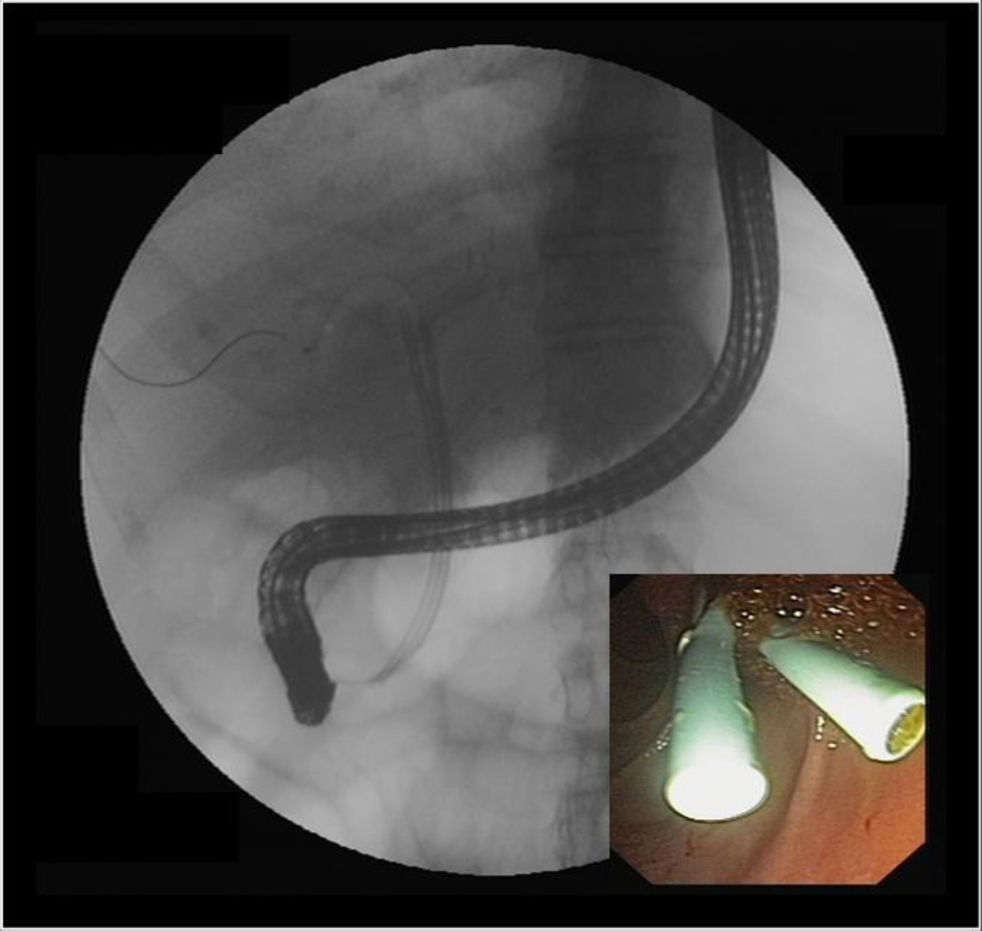
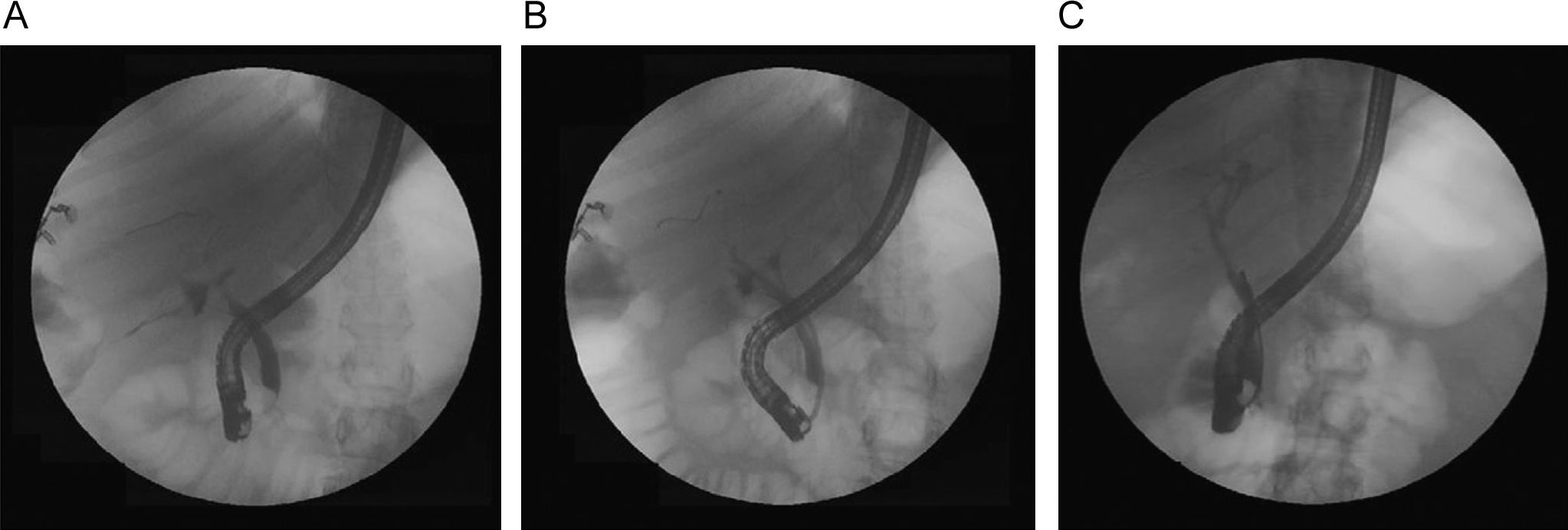
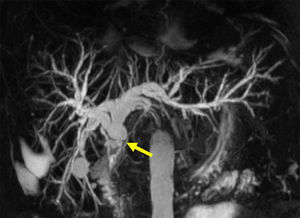
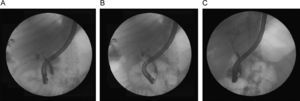
Anastomotic stricturesAS represent about 80% of biliary strictures after LT.4 They are single and short with a characteristic cholangiographic appearance (Figures 3 and 4). Factors related to AS development are technical problems of the biliary anastomosis, prior anastomotic bile leaks, or ischemia. Management: Initial management is performed with ERC and biliary sphincterotomy. A key aspect of the endoscopic management of strictures is traversing the stricture with a guide-wire. Some patients require a prolonged session using different types of soft-tipped guide-wires in order to achieve success. In difficult cases the use of peroral cholangioscopy might be helpful given that the stricture can be directly visualized; this may avoid long procedures.28,29 If the endoscopist cannot get the guide-wire to traverse the stricture, a percutaneous internal-external drainage placed with PTC should be performed. This allows therapy by the percutaneous route and may also be used to perform endoscopic therapy with a combined (PTC plus endoscopy) rendez vous technique in a second attempt30 (Figure 2). Once the guide-wire is in the proximal biliary tree, balloon dilation of the stricture (diameters of 6–8mm) is performed followed by placement of plastic stents (in DDLT: 7–11.5 Fr stents and in LDLT: 7–8.5 Fr stents).31 Patients with AS usually require several ERC sessions every 3 months and long-term stenting (for 12–24 months). Of interest, a recent case series found no difference using only balloon dilation versus standard therapy, however the study included a small number of patients.32 Plastic stents should be exchanged at 3-month intervals to avoid stent occlusion and cholangitis. The placement of a progressively increasing number of stents with each subsequent ERC has been shown to be a successful method of treating AS33,34 (Figure 5). In general most patients require between 3 and 5 sessions in order to achieve resolution of the stricture. With this approach long-term success rate range between 70% and 100%.1,14,25,26,31–33,35 After a successful endoscopic treatment (balloon dilation and stenting) the recurrence rate is 18% in DDLT36 and up to 33% in LDLT.37 The presence of anastomotic bile leak associated to the stricture is a factor that influences biliary stricture recurrence after successful endoscopic therapy.36 A recent case series of 16 patients showed a clear benefit using temporary (2 months) placement of a completely covered self-expanding metal stent as rescue treatment in patients with failure or recurrence of AS after balloon dilation and stenting.38 These stents may occlude secondary branch ducts, limiting their use in AS in patients with LDLT. However data is very limited and more information is needed in order to consider the use of covered stents in AS after initial endoscopic therapy failure.
In summary, patients with duct-to-duct anastomosis and AS should undergo ERC first before considering percutaneous interventions or surgical repair. In patients with Roux-en-Y choledochojejunostomy, management with balloon enteroscopy ERC or PTC and dilation followed by placement of a percutaneous transhepatic catheter are often necessary. Surgical intervention (usually a repair or conversion to a Roux-en-Y choledochojejunostomy) is required when the ERC or PTC fails to adequately treat AS.
Nonanastomotic stricturesNonanastomotic strictures result mainly from hepatic artery complications causing peribiliary arteriolar endothelial injury and irreversible biliary injury.15 Other factors such as long cold ischemia time or ABO-blood type incompatibility may be implicated in the pathogenesis. NAS account for 10–25% of all biliary strictures after LT, with an incidence that ranges in 0.5–10%.1–4 The cholangiographic appearance of NAS resembles primary sclerosing cholangitis. Involvement of the bile duct proximal to the anastomosis and the intrahepatic branches is a common feature. Biliary sludge can accumulate proximal to the strictures leading to the formation of casts favoring infections.39 NAS tend to occur earlier than AS, with a mean time to stricture development of 3–6 months.4,5
Management: endoscopic treatment of NAS is more difficult and less successful than that of AS. The endoscopic approach is similar and as with AS and consists of sphincterotomy, balloon dilation (4–6mm compared with 6–8mm for AS), placement of plastic stents (10–11.5 Fr) with replacement every 3 months.3,33 Patients with NAS need a higher number of interventions than patients with AS to achieve resolution with ERC therapy.5 The outcomes of NAS are not favorable as they are for AS. Only half of patients have a long-term response with endoscopic therapy.3,5,14,40,41 Furthermore, up to 50% of patients undergo retransplantation or die as a consequence of this complication despite endoscopic therapy.3,4,8,16 As a general rule, ischemic events that lead to NAS with diffuse intrahepatic bile duct strictures are associated with poor graft survival and may require retransplantation in suitable candidates.
Bile leaksThe incidence of biliary leaks after LT ranges between 2% and 25%.1–4 Bile leaks may arise from the anastomosis, the cystic duct remnant, the T-tube tract, or from the cut surface of the liver (in the case of living donor LT). Many bile leaks can be resolved nonoperatively with early intervention.1–4
Bile leaks have been classified as early or late depending on if they occur before or after 1 month from the LT. Early bile leaks usually occur at the anastomotic site and are often related to technical issues, not to the type of biliary reconstruction. Factors that predispose grafts to early bile leaks include lack of perfusion from the hepatic artery and other technical problems. Late bile duct leaks usually are related to the removal of the T-tube, resulting from delay in T-tube tract maturation. The clinical presentation of bile leaks is variable. Some patients are asymptomatic and a fluid collection is incidentally found on abdominal ultrasound. Patients with a bile leak associated with T-tube removal may develop an acute, sharp, and persistent abdominal pain with or without peritoneal signs. Patients with late bile leaks present a higher probability of later biliary strictures compared to those with early leaks.7
Management:. Patients with ascites and severe abdominal pain and peritoneal signs should be promptly evaluated for surgery given that these patients are at risk of developing shock or sepsis if not intervened soon. Patients that undergo surgical exploration with lavage, drainage and correction of the leakage usually respond well.
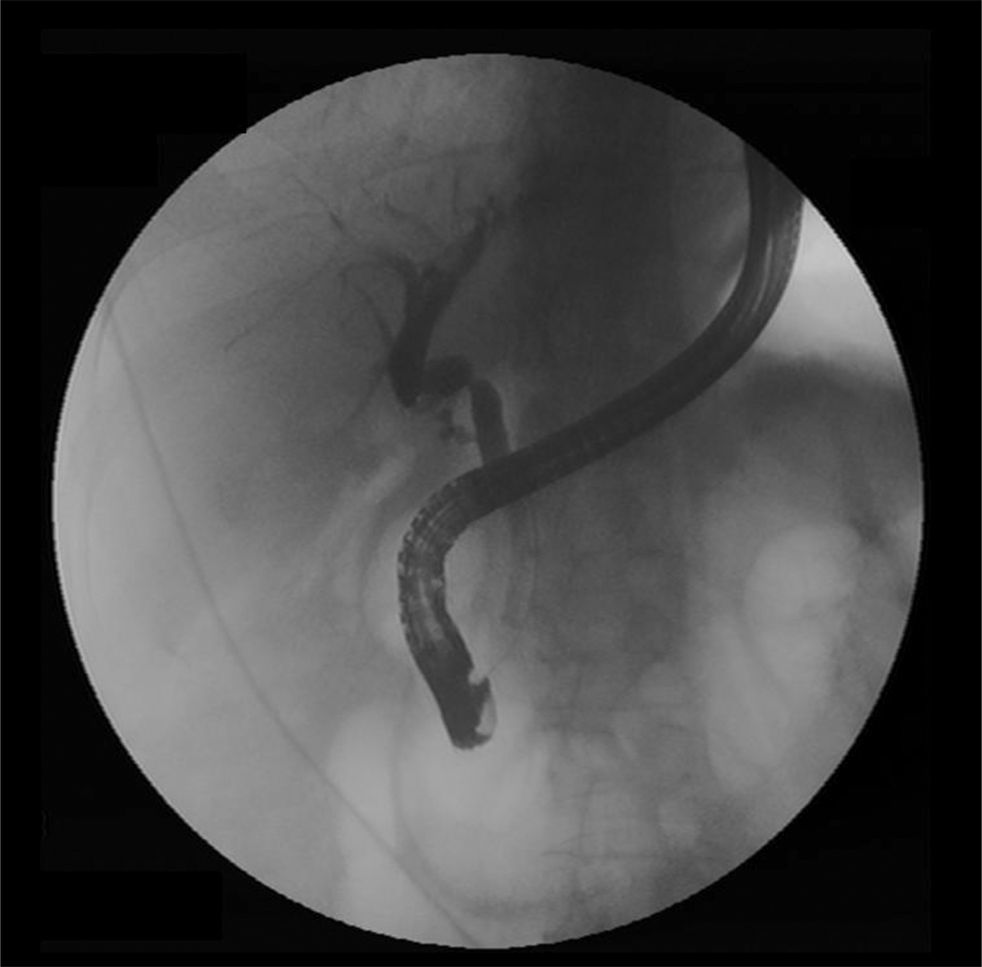
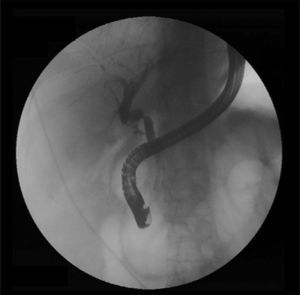
If there is a low suspicion of a biliary leak, sometimes a radionucleotide (HIDA) scan may be helpful as it has a reasonable accuracy in the noninvasive detection of a bile leak.42 However, ERC is considered the gold standard diagnostic method and should be performed in all patients when there is a high suspicion of a bile leak. Treatment of biliary leaks consists of a small biliary sphincterotomy and placement of plastic stent (Figure 6). A percutaneous drain is often necessary in patients with a large amount of ascites or biloma (Figure 1). The stent should be left in place for approximately 2 months because of problems with delayed healing that may arise due to immunosuppression.5 With the above approach approximately 90% of patients achieve resolution of the leak. Although clinical improvement occurs within a few days, complete resolution of the leak occurs in about 5 weeks.35 In patients who have a T-tube in place, small anastomotic leaks can be diagnosed with a T-tube cholangiogram and be managed by leaving the tube open without further intervention. Roux-en-Y choledochojejunostomy anastomotic leaks are less common. Standard ERC is often not feasible due to anatomical difficulties in reaching the biliary anastomosis except in centers with experience.23 Management is usually performed with percutaneous internal-external drainage or surgery.
Similar to biliary strictures, the use of temporary placement of covered self-expanding metal stent may have a benefit as rescue treatment for persistent bile leaks in DDLT patients previous to surgery.38 Although initial results are promising more data are needed in order to consider the routine use of these stents for this complication.
BilomasBilomas occur due to bile duct rupture and extravasation of bile into the hepatic parenchyma or the abdominal cavity. Most post-LT bilomas occur in the perihepatic area, outside of the liver. Large bilomas not communicating with the bile ducts are usually treated percutaneous drainage and antibiotics. Surgery is indicated when the patients have tense ascites, peritoneal signs or if the bile leak cannot be controlled effectively with the above measures.
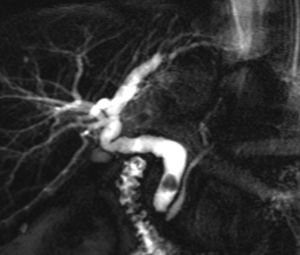
Biliary filling defectsBiliary filling defects occur in approximately 5% of patients after liver transplant. These defects may be due to gallstones, sludge, debris, blood clots, and casts. However about 70% of such defects are caused by stones (Figure 7).3,14 Symptoms in patients with common bile duct stones vary from asymptomatic cholestasis, to abdominal pain, or recurrent attacks of cholangitis. Biliary strictures, bacterial infections, ischemia (history of hepatic artery thrombosis or a prolonged cold ischemia time), and elevated total cholesterol and triglyceride levels can predispose a patient to the formation of biliary stones or sludge.5,43,44 Additionally stones may be present in the proximal bile duct of an AS in a significant amount of patients. Management is similar as with the nontransplant patients (biliary sphincterotomy and balloon or basket extraction) with a high rate of success rate in a single ERC therapeutic session44 (Figure 2). Following stone extraction there is approximately a 17% recurrence rate within a median of 6 months.5,23
Biliary cast syndrome is the presence of multiple casts within the biliary system that takes the physical shape of the bile ducts. The disorder occurs in 2.5–18% of liver transplant recipients and is associated with an increased morbidity, mortality, and incidence of rejection.43,45 The pathogenesis of the biliary cast syndrome is unknown but it is believed that ischemic factors and biliary strictures play an important role in the development of the syndrome.43,45 Typically it occurs within the first year after transplant. Analysis of the casts has shown that bilirubin is the primary element along with collagen, bile acid, and cholesterol. Clearance of casts is successful in 60% of patients using endoscopic and percutaneous methods.45 However, various combinations of sphincterotomy, balloon and basket extraction, stent placement, and lithotripsy are often necessary. Surgery is offered only when percutaneous and endoscopic methods are not successful. Unfortunately it has been reported that up to 22% of patients with biliary cast syndrome will require a retransplantation.45
Sphincter of Oddi dysfunctionSphincter of Oddi dysfunction (SOD) has been described in 2–7% of patients who undergo LT.1–3 SOD in LT recipients is defined as the presence of a dilated bile duct without stenosis or filling defects along with cholestasis. Manometry of the sphincter is typically not performed in these patients due to the high risk of complications, mainly pancreatitis. It is postulated that in the post-transplant setting, denervation of the distal common bile duct (ampullary region) secondary to surgical intervention may produce a hypertonic sphincter causing SOD. Compared to nontransplant patients, abdominal pain is not commonly present. Biliary sphincterotomy is the endoscopic treatment of choice with high success rate (80–100%).1–3 Although SOD in the nontransplant setting is a well-known risk factor for post-ERC pancreatitis, in transplant patients this situation is probably not similar as manometry is not routinely performed.
Living donor liver transplantationLDLT typically involves a single donor lobe (usually the right) that is transplanted into the recipient and an anastomosis is constructed from donor's right hepatic duct to the recipient's common bile duct. LDLT is associated with a higher incidence of biliary complications. The incidence of biliary strictures ranges between 12% and 40% and the rate of bile leaks ranges between 7% and 67%.46 An increased incidence of biliary strictures is associated with a technically more difficult anastomosis (multiple bile ducts), hepatic artery stenosis, bile leak, long duration of surgery, donor age greater than 50, and a MELD score greater than 35.47–49 Risk factors include a donor with three or more bile ducts, a recipient diagnosis of hepatitis C, and the experience of the transplant center at performing LDLT.50 The incidence of biliary strictures and biliary leaks decreases significantly once a center has performed more than 40 LDLT.50
Endoscopic management in LDLT recipients may be quite difficult due to the complex nature of the duct-to-duct reconstruction. Patients will often require frequent ERCs with the use of smaller caliber stents (7–8.5 Fr). ERC with balloon dilatation is successful in up to 65% of patients.46,49 Failure of a primary ERC with dilatation is associated with the appearance of late biliary strictures over 24 weeks from LT and more than 8 weeks between a 2-fold increase in serum alkaline phosphatase.49 The relapse rate of strictures is up to 30% and occurs more in patients with shorter duration of stenting.49
Complications of ERC in liver transplant patientsThere is vast experience with ERC in transplant recipients and in general it is considered a safe procedure. Nonetheless a small percentage of complications including pancreatitis, bleeding, infection or perforation may occur. According to different series in LT recipients, complications may develop in up to 10% of patients,2,3 which is similar to general population. The most common complications are pancreatitis, cholangitis, and post-sphincterotomy bleeding. In contrast to the nontransplant setting the main risk factors for ERC complications are mainly patient-related rather than procedure-related.51 ERC complications in LT recipients occur more commonly in those receiving sirolimus/everolimus therapy and those with renal failure.51,52
SummaryBiliary complications are common in recipients of deceased donor and live donor liver transplants and a high index of suspicion should lead to a rapid diagnosis with noninvasive testing. An abdominal US is the first step in the evaluation of patients with asymptomatic cholestasis. If there is a strong clinical suspicion of biliary pathology, the patient should proceed directly to ERC while a MRCP may be preferred if the index of suspicion for bile duct abnormalities is low. ERC is the initial procedure of choice for the management of most biliary complications after LT. Nonetheless other options such as PTC and surgery are effective if ERC is not successful or cannot be performed. A team approach for the management of these complications is recommended as they often can be difficult to manage thus the whole transplant team including endoscopists and radiologists need to work closely in order to treat these lesions in an expedient manner.
Conflict of interestNone declared.
Part of the research reported in this article was funded by the Emili Letang grant from the Hospital Clínic of Barcelona.