The introduction of noninvasive liver stiffness (LS) determination has heralded a new stage in the diagnosis and treatment of liver fibrosis.
AimWe evaluated the effect of food intake on LS in patients with different degrees of liver disease.
Patients and methodsWe evaluated 24 patients (F≤1, n=11 and F> 1, n=13). LS (Fibroscan®) and portal blood flow (PBF) (Doppler ultrasound) were studied before and 30min after ingestion of a standard liquid meal.
ResultsFood intake increased PBF (51±10%, p<0.001). Splanchnic hyperemia was accompanied by a significant rise in LS (from 7.8±3.3 to 10.3±4.1kPa, p<0.001). These increases were similar in patients with minimal fibrosis(F≤1) and in those with more advanced fibrosis or cirrhosis (F>1). Hemodynamic and LS values returned to baseline pre-meal levels within 2hours.
ConclusionLS increases markedly after ingestion of a standard meal, irrespective of the degree of fibrosis. Our results strongly suggest that LS should be measured in fasting conditions.
El desarrollo de nuevos métodos que permiten la determinación no invasiva de la rigidez hepática ha abierto una nueva era en el manejo de la fibrosis hepática.
ObjetivoEl objetivo del trabajo fue evaluar el efecto de ingesta de una comida sobre la rigidez hepática en pacientes con diferentes grados de fibrosis.
Pacientes y métodosSe evaluaron 24 pacientes (F≤1, n=11, y F>1, n=13), que fueron estudiados basalmente y 30min después de la ingesta de una comida estándar (Ensure Plus®). La rigidez hepática se midió por Fibroscan®, y los parámetros hemodinámicos portales, mediante Doppler. La ingesta de una comida ocasionó un aumento del flujo sanguíneo portal (51±10%, p<0,001). La hiperemia esplácnica fue acompañada por un marcado incremento en la rigidez hepática (7,8±3,3 a 10,3±4,1kPa, p<0,001). Este efecto fue similar en pacientes con fibrosis mínima (F≤1) y con fibrosis significativa (F>1). Los valores de ambos parámetros retornaron a niveles similares a los basales a las 2h luego de la ingesta.
ConclusiónEste estudio demuestra que la respuesta vascular posprandial se acompaña de aumento de la rigidez hepática. Los cambios son independientes del grado de fibrosis. Nuestros resultados sugieren fuertemente que los estudios deben realizarse en condiciones de ayuno.
The medicine of the future is addressed, in addition to increasing the therapeutic efficacy to improve the quality of life of patients. It is for this reason that the use of less invasive tests, faster, easier to perform and with good sensitivity and specifity is highly recommended. In this regard, a new technique based on the evaluation of liver elasticity, called transient elastography, has been developed during the last years.1 This technique has demonstrated an excellent ability to exclude cirrhosis and it is good at identifying patients with different stages of fibrosis.2,3
From a physical point of view, the liver is an organ whapped in a distensible but non extensible Glisson's capsule, so stiffness is definitively influence by pressure that can be either hydrostatic or osmotic. This is evident in different clinical situations, such as inflammation, extrahepatic cholestasis, or congestion and it can interfere with measurements of liver stiffness, independently of fibrosis.4
Hemodynamic responses to feeding have been extensively studied in normal subjects and in patients with cirrhosis.5,6 In these studies, a postprandial hyperemia occurs in the splanchnic vascular bed following ingestion of the meal.5,6 Considering the dynamic component of the liver stiffness and splanchnic vasodilatation associated with food intake, we observed that the fasting condition has not been considered in most studies. Moreover, in a study of Castera et al., the authors said that the examinations were performed on a non fasting condition.7 Therefore, the aim of the present study was to determine if the measurement of liver stiffness is altered after food intake in patients with different degrees of liver disease.
Patients and methodsWe prospectively studied 24 subjects with different degrees of liver disease. They were referred to the liver center for the study of abnormal liver function tests, specifically increased transaminases. All the patients included had had liver biopsies. The degree of liver fibrosis was established based on the Metavir score.
The protocol was approved by the Clinical Research Committee of the Hospital Aleman in July 2012. Subjects gave their consent to participate after a full explanation of the nature and purposes of the study.
Blood flow measurementBlood flow was measured by a duplex scanner,8–10 comprising a real-time, two dimensional, ultrasonic scanner and an associated 3.5MHz pulsed Doppler flowmeter. After a sampling marker had been set in the middle of the lumen (portal vein) along the beam axis, a second marker was positioned parallel to the direction of blood flow. Care was taken to maintain the angle ϴ (theangle formed by the ultrasonic beam and blood flow direction) below 60°, since the accuracy of the measurements decreases with greater angles. Every measurement was repeated until good and reproducible spectrum patterns and blood sounds were obtained. Measurements were carried out during expiration, because it can be easily be standardized and permits a better visualization of the portal vein for Doppler purposes as the angle ϴ is reduced to a minimum.
Liver stiffness measured by FibroscanDetails of the technical description and examination of the procedure have been previously described.1–4 The tip of the probe transducer was placed on the skin between the ribs and the level of the right lobe of the liver. The measurement depth was between 25 and 65mm. Ten measurements were performed with success rates of at least 60%. The results were expressed in kilopascals (kPa). The median value was taken as representative.
After an overnight fast and a resting period of 15-20min in a supine position, both parameters were measured. Measurements were obtained in baseline conditions and 30min after the administration of 330ml of Ensure Plus (Ross
Laboratories, Columbus, Ohio). This product is a Iiquid meal supplying 13.09 of protein, 12.69 of fat and 47.39 of carbohydrate per 100g. Measurements were performed in triplicate in each period of the study. Doppler evaluation and liver stiffness measurements were performed by the same specialized examiner (DA).
The results were expressed as mean±standard deviation (SD). Statistical analysis of the results was performed using unpaired Student's test and the analysis of variance (ANOVA). Significance was considered at p<0.05.
ResultsTwenty four patients with different degrees of liver disease were analyzed. There were 13 males and 11 females, and the mean age was 53±10 years. The most common cause of liver disease was hepatitis C (66%, CI 44-84). All the patients had prior liver biopsies in order to stage the degree of liver disease which showed that 11 patients (45.8%, CI 25-67) had being classified as F≤1 and 13 patients (54%, CI 32-74) as F>1 based on the Metavir score. The baseline characteristics of patients are presented in Table 1.
Main clinical and biomedical characteristics of patients included in the study (n=24).
| Age (years) | 53±10 |
| Male (%) | 13 (54) |
| Etiology | |
| HCV (Liver transplant) | 16 (4) |
| Hemochromatosis | 2 |
| Autoinmune diseases | 3 |
| Others | 3 |
| *ALT (UI/L) | 75.8±65 |
| *AST (UI/L) | 62.5±51 |
| *FAL (UI/L) (x normal value) | 1.3±0.6 |
| *Total bilirrubin (mg/dl) | 1.3±0.9 |
| Liver fibrosis (Metavir score) | |
| F0-F1 | 11 |
| F2-F4 | 13 |
*Mean±SD.
Food intake caused a significant increase of portal venous blood flow in every patient studied. As previously described, the maximum effect was observed 30min after food intake.5 This time was chosen to express both hemodynamic and liver stiffness changes. The increase in portal blood flow, from 1026±596 to 1546±780ml/min, p<0.001, was entirely due to a significant rise in mean blood velocity without changes in the caliber of the portal vein. No significant changes were observed in mean arterial pressure and hear rate.
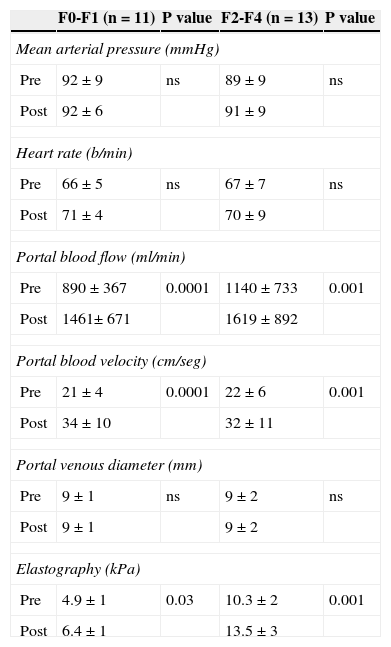
The splanchnic hyperemia observed after standard meal ingestion was accompanied by a significant increase in liver stiffness (from 7.8±3.3 to 10.3±4.1kPa, p<0.001), (Fig. 1). It is noteworthy that this effect on both parameters was similar in patient with a liver with minimal fibrosis (F≤1) than those with more advanced fibrosis or cirrhosis (F>1) (Table 2). No significant correlation was observed between changes in portal flow and liver stiffness (Rho -0, 14, p= 0.52).
Hemodynamic and liver stiffness changes after a meal ingestion in the studied population of patients (n=24).
| F0-F1 (n=11) | P value | F2-F4 (n=13) | P value | |
|---|---|---|---|---|
| Mean arterial pressure (mmHg) | ||||
| Pre | 92±9 | ns | 89±9 | ns |
| Post | 92±6 | 91±9 | ||
| Heart rate (b/min) | ||||
| Pre | 66±5 | ns | 67±7 | ns |
| Post | 71±4 | 70±9 | ||
| Portal blood flow (ml/min) | ||||
| Pre | 890±367 | 0.0001 | 1140±733 | 0.001 |
| Post | 1461± 671 | 1619±892 | ||
| Portal blood velocity (cm/seg) | ||||
| Pre | 21±4 | 0.0001 | 22±6 | 0.001 |
| Post | 34±10 | 32±11 | ||
| Portal venous diameter (mm) | ||||
| Pre | 9±1 | ns | 9±2 | ns |
| Post | 9±1 | 9±2 | ||
| Elastography (kPa) | ||||
| Pre | 4.9±1 | 0.03 | 10.3±2 | 0.001 |
| Post | 6.4±1 | 13.5±3 | ||
Mean±SD.
Fig. 2 shows the effect of food intake in 6 patients with chronic hepatitis C where basal liver stiffness values were normal. In this subgroup of patients a marked increase in liver stiffness after food intake was observed (+ 32%, p<0.05).
Finally, in 6 additional subjects we have evaluated the time necessary for the normalization of portal blood flow and liver stiffness after food intake. As shown in Table 3, two hours after meal ingestion, portal blood flow and liver stiffness values were similar to those obtained at baseline conditions.
Portal blood flow and liver stiffness data at baseline and after food intake in 6 additional subjects. Note, that the food intake increases both parameters in each of the subjects studied. These values returned near baseline after two hours of having the food intake.
| Baseline | Food intake | Two hours after food intake | |
|---|---|---|---|
| Portal blood flow (ml/min) | 910±280 | 1128±340 | 990±320 |
| Liver stiffness (kPa) | 6.9±3.2 | 9.1±3.8 | 6.8±3.0 |
Mean±SD.
Noninvasive diagnosis of liver fibrosis is an area that has developed very rapidly in recent years. In this regard, transient elastography has been becoming the most widely used noninvasive method for assessing the degree of liver fibrosis. Moreover, this technique has demonstrated a potential role in the evaluation of clinical outcomes, based on the correlation with portal pressure, which is a very good predictor of clinical events.2,11 Finally, transient elastography was also useful in assessing the severity of HCV recurrence in patients who have undergone transplantation.12
The results of our study clearly show that the splanchnic vasodilatation associated with the ingestion of a standard meal is accompanied in every patient studied by a significant increase in liver stiffness. From the clinical point of view, the most important message of this study is the fact that, to avoid overestimation in this group of subjects, determinations should be performed at least two hours fasted.
Despite that the Fibroscan® has been introduced several years ago, some basic methodological aspects had not been well defined. In this regard, recent studies have been aimed at evaluating whether the ingestion of a standard meal produces changes in liver stiffness.13–15 Mederacke et al. were the first to show that liver stiffness values could be affected after a meal.13 More recently, Berzigotti et al. and Arena et al., show that the liver stiffness significantly increases after a liquid test meal.14,15 This effect was observed in patients with cirrhosis and portal hypertension and in subjects with varying degrees of fibrosis. In the latter study, the increase became more pronounced with increasing fibrosis stages and was maximal in cirrhosis, with media stiffness differences ranging from 1.9kPa in F0-F1 fibrosis to 4.7kPa in cirrhosis.15
Liver stiffness, like any other soft tissue stiffness, is composed of vivo components: static one, due to the extracellular matrix of the organ, in this case the liver, and a dynamic linked to hydrostatic and osmotic pressure. Therefore, in patients with chronic liver disease the increase in liver stiffness may be related to both components. In our study, the rise in liver stiffness after food intake did not correlate with the increase in the portal blood flow (Fig. 2). An additional information was recently reported by Bazigotti et al. 14, These authors showed that patients who had a reduction of hepatic artery blood flow (- physiological response to increased portal blood flow after meal-) had a significantly lower increment of the liver stiffness compared to patients in whom hepatic artery blood flow increased post-prandially.14 This reasoning was based on the fact that liver stiffness changes showed a direct and significant correlation with changes in hepatic artery blood flow in cirrhotic patients.
In recent years, clinical practice guidelines have become an essential tool in the process of decision-making. Therefore, and prior to the incorporation of a new diagnostic method, as in the case of transient elastography, it is necessary to identify factors that may affect it. Until the appearance of the work of Mederacke et al., the vast majority of studies to evaluate the liver stiffness were performed in non fasting conditions.1–4 Is probably that, a number of false positive liver stiffness measurements might have been due to food intake prior of the determinations in a number of patients. To reinforce this concept, in our study we evaluated the effect of food intake in a subgroup of HCV patients with early stages of fibrosis (i.e. F0-F1). An overestimation of 2-3kPa was observed, this can have a significant impact on the interpretation of the liver stiffness measurements, and most important, producing an error in the clinical management of these patients.
In conclusion, our study clearly shows that liver stiffness increases after a liquid food intake in subjects with different degrees of fibrosis. In order to optimize this non invasive method, all measurements should be always performed with the subjects at least two hours fasted.