A 40 year-old man was referred to the Hepatology out-patient clinic due to the detection of abnormal liver blood tests. He had a history of acute lymphoblastic leukemia requiring standard chemotherapy and an autologous stem-cell transplantation, achieving complete remission. He did not smoke, nor consume alcohol or any illicit drugs. He did not take any prescribed medication. Additionally, he did not report any liver disease, neither in his family. Previous laboratory tests throughout these years showed normal liver enzymes.
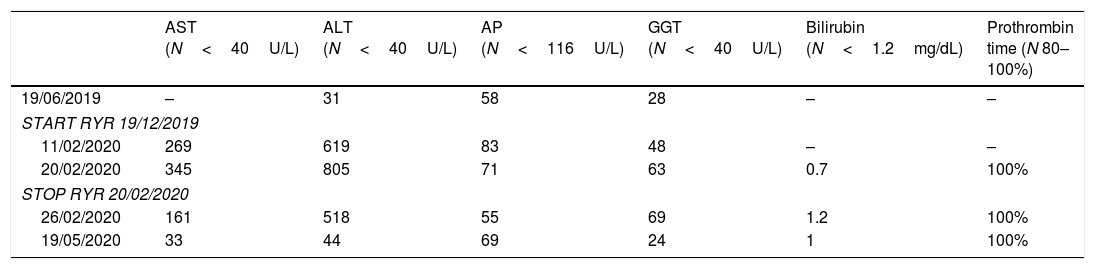
During a follow-up visit in December 2019 high cholesterol levels were detected, and the patient was told to start statins. However, he rejected the prescribed drug and decided to start over-the-counter red-yeast rice (RYR) 600mg/day (Rice Plus Q10 Nadiu©, Spain). A month later, the patient developed progressive weakness and tiredness, which finally lead to a consult to the general physician. Physical examination was unremarkable. Laboratory tests revealed marked hypertransaminasemia with normal GGT, alkaline phosphatase, total bilirubin, prothrombin time and albumin (Table 1). Creatine kinase levels, renal function and complete blood count were within the normal range as well. A week later, laboratory tests showed further increase in the transaminases levels and the patient was instructed to stop RYR pills and transferred to our Liver Disease Clinics.
Evolution of liver enzymes.
| AST (N<40U/L) | ALT (N<40U/L) | AP (N<116U/L) | GGT (N<40U/L) | Bilirubin (N<1.2mg/dL) | Prothrombin time (N 80–100%) | |
|---|---|---|---|---|---|---|
| 19/06/2019 | – | 31 | 58 | 28 | – | – |
| START RYR 19/12/2019 | ||||||
| 11/02/2020 | 269 | 619 | 83 | 48 | – | – |
| 20/02/2020 | 345 | 805 | 71 | 63 | 0.7 | 100% |
| STOP RYR 20/02/2020 | ||||||
| 26/02/2020 | 161 | 518 | 55 | 69 | 1.2 | 100% |
| 19/05/2020 | 33 | 44 | 69 | 24 | 1 | 100% |
Laboratory work-up excluded acute viral hepatitis (HAV, HBV, HCV and HEV), auto-antibodies were negative and IgG levels were within the normal range. Abdominal ultrasound was unremarkable. CIOMS/RUCAM score for RYR was 8 points (probable), therefore, diagnosis of drug-induced liver injury (DILI) due to RYR was established.
Just a week after discontinuing RYR the patient referred improvement in his general condition and laboratory tests revealed a decrease of transaminases levels, which returned to normal levels 3 months later (Table 1).
DiscussionHerbal and dietary supplementation (HDS) are commonly used around the world, either in association or in substitution of prescribed drugs. Importantly, patients assume that these products are safe and their use is generally over-the-counter without any medical supervision. The increasing use of such products, the lack of rigorous surveillance in their preparation and marketing and the low awareness of their potential liver toxicity by physicians are relevant problems. Indeed, drug-induced liver injury (DILI) represents one of the most frequent causes of acute liver failure in Western countries,1 and some studies have showed that almost 10% of all DILI-related acute liver failure are due to HDS.2
RYR is a traditional Chinese medicine used to improve digestion and also as a revitalizing agent. In addition, it has been shown to reduce cholesterol levels and thus, its use has been popularized in recent years as a natural way for treating hypercholesterolemia. RYR is produced by the fermentation of white rice by the yeast Monascus purpureus. Its active agent is monacolin K, a secondary metabolite with cholesterol-synthesis inhibiting properties because of its identical chemical structure to lovastatin.3 Thus, although RYR is claimed to be a safer alternative to regular statins, structural similarity with lovastatin implies that similar adverse reactions can be expected. Furthermore, monacolin content is not standardized among marketed products and are generally not depicted on labels.4 Indeed, a study assessing monacolin content in 12 commercial RYR formulations labeled as “600mg/capsule of active product” found marked variability, ranging from 0.10 to 10.09mg of monacolin per capsule, challenging the efficacy and safety of the product.5 In our case, the label stated that monacolin K content was at least 10mg per capsule.
The U.S. Food and Drug Administration (FDA) has ruled that RYR with more than trace amounts of monacolin K cannot be sold as dietary supplements, which is not the case of the European Food Safety Authority (EFSA).
There are isolated case-reports of over-the-counter RYR hepatotoxicity, all of them are mild-to-moderate in severity and self-limited in course, which are consistent with the one presented in this report. For this reason, taking into account that HDS are easy-to-obtain products, considered safe for most people, contain multiple active ingredients in poorly specified amounts and with incomplete labeling, safety can very well be compromised. Therefore, physicians must remain alert that HDS might be the cause of acute liver injury in some patients.
Conflict of interestXF has acted as advisor for Abbvie and Gilead. GS has nothing to report.