The current goals of treatment in inflammatory bowel disease, both Crohn's disease and ulcerative colitis, are to achieve clinical, endoscopic and ideally histological remission and improve the quality of life of these patients. Current therapies are effective in achieving remission in most cases, but there is a lack of clear guidelines on their optimal duration. This review aims to evaluate the current evidence on the withdrawal of therapy with 5-aminosalicylates, thiopurines and methotrexate. We also aim to identify which specific group of patients, while in remission and in the absence of risk factors, may be able to discontinue therapy without a significant risk of relapse.
Los objetivos actuales del tratamiento en la enfermedad inflamatoria intestinal (EII), tanto en enfermedad de Crohn como en colitis ulcerosa, son alcanzar la remisión clínica, endoscópica e idealmente histológica, mejorando de esta manera la calidad de vida de estos pacientes. Las terapias actuales son efectivas en lograr estos objetivos, pero no existen guías claras respecto de la duración óptima del tratamiento de mantención. Esta revisión tiene por objetivo evaluar la evidencia actual respecto del retiro de la terapia con 5-aminosalicilatos, tiopurínicos y metotrexato. A su vez, buscamos determinar grupos específicos de pacientes que, encontrándose en remisión y en ausencia de factores de riesgo, pudieran suspender la terapia con el menor riesgo de recaída posible.
Inflammatory bowel disease (IBD), essentially Crohn's disease (CD) and ulcerative colitis (UC), is a group of immune-mediated disorders affecting the gastrointestinal tract, with alternating periods of activity and remission. IBD generally occurs in middle-aged people and can compromise their ability to carry out their usual activities and have a significant impact, both financially and on their work life.1,2
There are no cures available for the time being, and the aim of treatment is therefore to achieve control of the disease, keeping it in remission and relapse-free, to allow patients a better quality of life.3,4
The drugs used to treat IBD have been studied and tested for their efficacy. Treatment is long-term or permanent, and side effects and costs can often become a limitation or have a negative effect on patient adherence.5
In this context, a number of studies have sought to examine the optimal duration of the different therapies and the possibility of discontinuing them at some point. The aim of this review was to present the available evidence on the possibilities of discontinuing maintenance therapy once remission has been achieved, indicating the factors that need to be considered prior to withdrawal of the drug, with personalised stratifying of the risk of relapse in each patient.
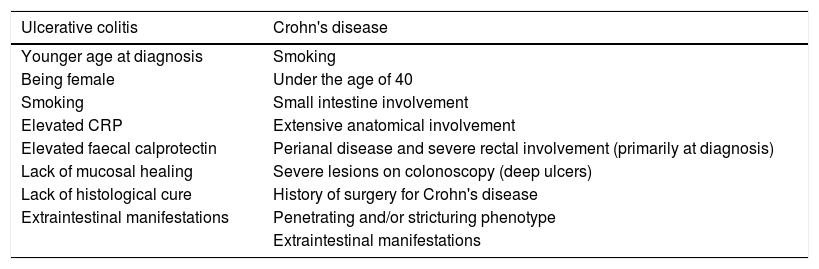
Discontinuing treatment in inflammatory bowel diseaseWhen deciding whether or not to discontinue therapy, it is important to first find out what the patient thinks about it, and then assess the risk factors associated with each specific patient, including demographic, clinical, laboratory, endoscopic and histological parameters.6 These factors will help us to make a decision by identifying which patients are more or less likely to respond unfavourably to the withdrawal of therapy (Table 1).
Risk factors associated with poor prognosis in patients with inflammatory bowel disease.
| Ulcerative colitis | Crohn's disease |
|---|---|
| Younger age at diagnosis | Smoking |
| Being female | Under the age of 40 |
| Smoking | Small intestine involvement |
| Elevated CRP | Extensive anatomical involvement |
| Elevated faecal calprotectin | Perianal disease and severe rectal involvement (primarily at diagnosis) |
| Lack of mucosal healing | Severe lesions on colonoscopy (deep ulcers) |
| Lack of histological cure | History of surgery for Crohn's disease |
| Extraintestinal manifestations | Penetrating and/or stricturing phenotype |
| Extraintestinal manifestations |
Source: Risk factors associated with inflammatory bowel disease.7–33
The risk factors for a more severe disease course in UC include:
Younger age at diagnosis. Being younger at diagnosis has been associated with shorter intervals between flare-ups, poorer response rate to drug treatment and a higher risk of colectomy.7–13 Cohort studies of paediatric patients report colectomy rates of up to 20% at five years after diagnosis compared to 8% at ten years after diagnosis in patients over the age of 60.14,15
The IBSEN study shows that patients diagnosed with IBD beyond the age of 50 have a 70% reduction in the risk of colectomy compared to patients diagnosed before the age of 30.7
Being female. A cohort study of 771 patients found that the women had a 1.2-fold higher risk of relapse than the men (p<0.001), although there was no gender relationship with the rate of colectomy.8
Smoking. Being an active smoker has been implicated as a protective factor in UC. Patients who continued to smoke had a greater regression of disease extent after five years of follow-up (p<0.001).16 Moreover, some studies show smokers to have a lower risk of requiring colectomy after ten years of follow-up.17 Caution needs to be exercised on this point, given that it is not advisable for patients to start smoking for therapeutic purposes. Nevertheless, when deciding about discontinuing one of the existing drug therapies, smoking should not be considered as a determining variable for this approach.
Elevated C reactive protein (CRP). It has been demonstrated that high levels of CRP are directly proportional to the extent of the disease at the time of diagnosis; with CRP >23mg/l associated with a nearly five-fold higher risk of colectomy (OR: 4.8; p=0.02).17–19
Elevated faecal calprotectin (FC). Values above 250μg/g are associated, with a sensitivity >70% and specificity close to 80%, with the degree of inflammation of the colonic mucosa.20
Mucosal healing. The consensus is that mucosal healing is the absence of friability, blood, erosions and ulcers in the colonic mucosa. In this context, the disappearance of the submucosal vascular pattern is considered as mucosal healing. Several studies have associated achieving this goal with a lower risk of relapse. The ACT1 and ACT2 studies in patients with UC with moderate to severe activity on maintenance therapy with infliximab found that 48.3% of patients who achieved mucosal healing after eight weeks of treatment maintained remission at week 30 compared to 9.5% in those who did not achieve mucosal healing after eight weeks.21 This factor has also been associated with a lower risk of colectomy. The IBSEN study showed that, regardless of the treatment received, patients who achieved mucosal healing after one year of treatment had a lower risk of colectomy (2%) compared to those not achieving mucosal healing after five years of follow-up (7%) (p=0.02).22
Histological cure. This has been taken as a marker of more profound disease control compared to mucosal healing. A study of 646 patients showed that patients who achieved histological normalisation (10% of the analysed population), which was defined as completely normal mucosa without even signs of chronicity, were independently associated with a higher probability of relapse-free survival compared to patients who only achieved histological quiescence (p=0.007), defined as absence of acute activity, but presence of chronic changes, or those who continued to have acute histological activity (p=0.001).23 However, these criteria are not widely validated and a standardised histological reporting system is necessary to unite criteria in this area.
In the case of CD, the risk factors associated with a worse prognosis include the following:
Smoking. Smoking is associated with an increased risk of developing early CD, requiring surgery and having post-surgical relapses.24–26
Age below 40at diagnosis has been associated as an independent risk factor for surgery (p=0.03).27–29
Small intestine involvement. This tends to have a poor response to therapy, leading to a more frequent need for surgery and nutritional support, with higher healthcare costs.29–31
Upper gastrointestinal tract involvement. This type of involvement at the time of diagnosis has been associated with an early need for complex abdominal surgery (HR: 5.7), with higher relapse rates.32
Extensive anatomical involvement. This is associated with greater nutritional deficiencies, more frequent complications, and, in the context of surgical resections, the development of short bowel, which is why the recommended management of choice in these phenotypes is strictureplasty.33
Perianal disease and severe rectal involvement (primarily at diagnosis). This has been associated with greater disease-related disability (OR: 4.340) and a higher rate of repeat surgical intervention (OR: 2.189).29
Severe lesions on colonoscopy (deep ulcers). Deep and extensive ulcers are associated with a higher colectomy rate (RR: 5.4). In a study that included a total of 102 patients with active ileocolonic CD, 52 had deep ulcers. At 52 weeks of follow-up, 58% of them required a colectomy compared to 12% in the group without deep ulcers.34
History of surgery for CD. This is a risk factor for surgical recurrence. Of patients who have had a surgical resection, 25% require a further resection at five years, and 35% at ten years.35 This factor has been more associated with penetrating disease, a greater number of previous operations, longer duration of the disease and active smoking.36
Penetrating and/or stricturing phenotype. Penetrating phenotype (HR: 8.6) or stricturing phenotype (HR: 9.4) at the time of diagnosis have been associated with a shorter time before the need for complex abdominal surgery. Both factors are also associated with higher relapse rates.32
Severe extraintestinal manifestations. The most common extraintestinal manifestations are large joint involvement, uveitis, iritis, episcleritis, erythema nodosum and pyoderma gangrenosum, which often impede and complicate the course of the gastrointestinal disease, making the treatment more complex.33
AminosalicylatesSulfasalazine and the 5-aminosalicylic acid (5-ASA) derivatives are the initial treatment strategy for mild to moderate UC.3,37–39 There are studies which show that these drugs are effective in preventing relapse in patients with UC after a prolonged period of remission. Studies involving discontinuation of 5-ASA are mostly from the 1970s and include small populations. Moreover, the definition of remission was based mainly on clinical criteria, without considering more stringent objectives (endoscopic, possibly histological, and/or biomarkers such as FC) when deciding whether to stop or continue with therapy.
A double-blind, randomised, controlled study assessed 112 patients with UC in clinical, endoscopic and histological remission on treatment with 5-ASA (1.2g/day) for at least one year. They were divided into two groups according to the length of clinical remission prior to randomisation: 61 patients in remission for 12–24 months (26 patients with 5-ASA and 35 with placebo), and 51 patients in remission for more than 24 months (28 patients with 5-ASA and 23 with placebo). In the first group, it was found that 5-ASA was more effective than placebo at preventing 12-month relapse (5-ASA 23% vs placebo 49%; p=0.035). In contrast, in the patients in remission for over 24 months, there was no statistically significant difference versus placebo in terms of 12-month relapse (5-ASA 18% vs placebo 26%, p=0.35). This study shows that it is possible to decrease the mesalazine dose once remission has been achieved for a period of at least 24 months.40 However, it is important to consider that the population studied is small and that the frequency and manner in which patients were followed up over time is not reported. In this context, it would seem necessary to carry out follow-up with FC every 3–4 months, with target values <250μg/g.41–43 One study of 49 patients in clinical remission for at least 12 months after maintenance therapy with sulfasalazine who were randomised to continue therapy with sulfasalazine (n=25) or placebo (n=24) found that the recurrence rates (rectal bleeding for more than three days or more than three daily bowel movements for five consecutive days) at the six-month follow-up were similar in the two groups (sulfasalazine 24% vs placebo 29%). There were also no differences between the patients in remission for 12–24 months and those in remission for more than 24 months, suggesting that the withdrawal of 5-ASA should be an option in patients in remission for more than 12 months.44 These results contradict those of the previous study where, in patients in remission for 12–24 months, mesalazine was more effective at preventing relapses than placebo.40
A double-blind, placebo-controlled study randomised 64 patients with UC who were in clinical, endoscopic and histological remission for at least one year after treatment with sulfasalazine 2g/day to continue with sulfasalazine or placebo (12). The 12-month relapse rate was 12.1% in the sulfasalazine group versus 54.8% in the placebo group (p<0.001).45
A review by Wang et al. included 41 randomised, controlled studies (8928 patients), which measured the effectiveness of maintenance therapy with 5-ASA for more than six months in patients with UC. Seven of the studies (n=1298) reported results in terms of failure to maintain clinical or endoscopic remission, comparing the response to 5-ASA versus placebo. They reported that 5-ASA therapy was superior to placebo in maintaining clinical and endoscopic remission, with 41% relapse in the 5-ASA group versus 58% in the placebo group (RR: 0.69; 95% CI: 0.62–0.77). Greater benefit was found in the groups with maintenance doses of 1–1.9g/day (RR: 0.65; 95% CI: 0.56–0.76) and >2g/day (RR: 0.73; 95% CI: 0.60–0.89).46
In view of the wide range of doses suggested as effective for maintaining remission, the minimum dose needs to be defined for each patient individually. Also, in addition to the clinical symptoms, monitoring with FC should be considered.
In patients with ulcerative proctitis, studies show that topical therapy is more effective than placebo in achieving clinical and endoscopic remission.38,39,47,48
Treatment with mesalazine suppository 1g single dose is equally effective and better tolerated than doses of 500mg administered twice a day.49,50 It has been suggested that, in patients with proctitis who have achieved clinical remission, the use of mesalazine suppositories three times a week may be considered.51 It is even feasible to discontinue 5-ASA suppositories if clinical remission is maintained and the FC is <50μg/g. This biomarker has been shown to have a good correlation with endoscopic activity in patients with ulcerative proctitis, and is useful in the follow-up of these patients.52 There is evidence that more than the extent of the disease, the best correlation is with the severity of the disease. Extensive UC is known to be associated with greater severity than proctitis. However, severe proctitis can be associated with very high FC values.20,53,54 Two studies state that the extent of UC has a significant association with FC values in the univariate analysis, but, after adjusting for the extent and severity of the disease activity, only the severity of the activity maintained a statistically significant association with the FC value.55,56
With regard to the utility of 5-ASA in patients with CD, a systematic review has shown that it would not be superior to placebo in maintaining remission.57 According to the latest recommendation of the European Society guidelines on the management of CD (ECCO 2017), 5-ASA does not have a beneficial effect over placebo, and is inferior to budesonide.58 In this scenario, it is plausible that, in a case of mild colonic CD, in remission with mesalazine, attempts could be made to gradually withdraw the drug, with serial monitoring of FC and possibly colonoscopy and imaging in order to identify the need to restart 5-ASA or possibly immunomodulatory therapy as a short-term maintenance strategy.37
The follow-up after therapy withdrawal or dose reduction should be both clinical and laboratory-based, with measurement of FC as a parameter for quantification of subclinical inflammation and predictor of relapse. D’Haens et al. suggest that there is a relationship between FC levels and endoscopic activity (Mayo Endoscopic Subscore [MES]), the extension and the clinical activity of the disease (Mayo Index). A cut-off of 250μg/g had sensitivity of 71% and specificity of 85.7% to show inflammation of any degree (MES 1–2 vs 0) and sensitivity of 100%, with a specificity of 77.8% to show moderate-to-severe inflammation (MES 2–3).20 Other authors have also recently confirmed the correlation of FC with the endoscopic and histological activity scores.59,60
In sum, there are factors for and against the withdrawal of 5-ASA. The risks of relapse versus the benefits of stopping therapy need to be considered, taking into account that mesalazine is a relatively low-cost, easy-to-use, well tolerated drug, which can even be administered in single doses, with very few adverse effects. This is the context in which the continuation or discontinuation of therapy should be considered, with the fact that there are now laboratory tools such as FC enabling us to monitor patients and point us towards the need to reintroduce treatment if relapse is suspected. We must not forget that the patient has to be included in any final decision and that they must be informed at all times of the risks and benefits of the chosen strategy.
Thiopurines: azathioprine–mercaptopurineThiopurines are antimetabolite prodrugs metabolised by three pathways to form the active metabolite, 6-thioguanine, which interrupts DNA replication of cells which are in constant replication, such as activated T lymphocytes.61 The most used in clinical practice are azathioprine (AZA) (dose 2–2.5mg/kg/day) and mercaptopurine (MP) (dose 1–1.5mg/kg/day). Because of the potential risk of leucopenia and hepatotoxicity associated with their use, there are laboratory methods that can anticipate the response of each patient to these drugs, and perform a posteriori follow-up in order to minimise adverse events. The current recommendation is to measure the activity of the enzyme thiopurine methyltransferase (TPMT) to prevent the risks of toxicity, start treatment with 50% of the dose per kg body weight and subsequently make dose adjustments according to the enzymatic activity of each patient and the laboratory test results (blood count and liver function tests).62 Thiopurines do not start acting until 8–12 weeks after the beginning of treatment, so they are not indicated in the induction of remission in either UC or CD.63–65 The measurement of AZA metabolites is useful to determine whether or not the dose is adequate or to assess adherence to treatment.62,66
The CESAME study cohort, in various publications, has shown the risks associated with prolonged use of thiopurines in patients with IBD, highlighting the development of lymphoproliferative disorders, non-melanoma skin cancer, myeloid disorders and urinary tract cancer. These risks have led clinicians to look into the feasibility of discontinuing these drugs once remission has been achieved for a prolonged period.67–70 A study in a French cohort of 844 IBD patients aged over 60 compared the incidence of cancer in this group with the database of the cancer registry in France (FRANCIM). The authors found that there was no significant increase in colorectal cancer for either UC or CD, but there was an increased risk of lymphoproliferative and myeloproliferative disorders in both populations. However, no association was found between the use of thiopurines and the development of these disorders (HR: 0.9).71
Although some older studies suggested that monotherapy with thiopurines could be suspended after three to four years of remission, subsequent studies have shown that there are high rates of relapse (65–87%) after withdrawal in patients with UC and CD.72–75 The Spanish Working Group on Crohn's Disease and Ulcerative Colitis (GETECCU) states that it would not be advisable to stop treatment with thiopurines, but, if it is stopped, close monitoring of disease activity is necessary, with early re-introduction of therapy in the event of a relapse.76
Thiopurines in ulcerative colitisThere have been very few studies of thiopurine discontinuation when used in monotherapy in patients with UC. A multicentre, prospective, double-blind, controlled study which included 79 patients with UC assessed the withdrawal of AZA. Patients who had been taking this drug for at least 6 months were randomised into two groups to continue AZA at the same dose as before or placebo. The one-year relapse rate was 36% in the AZA group versus 59% in the placebo group (HR: 0.5; 95% CI: 0.25–1.0) (p=0.039).77 Another multicentre, retrospective study which included 127 patients with UC in remission assessed the likelihood of relapse after withdrawing AZA. After discontinuation of AZA, with a median follow-up of 55 months, 35% of patients had relapsed at 12 months, 49% within the first two years, and 65% within the first five years.78
However, these results should be interpreted with caution in view of the heterogeneity in both the patient follow-up and how remission was defined when it was decided to withdraw therapy. What they do agree on is the high relapse rate, which was around 30% at two years and 50–75% at five years after stopping treatment.73,75 When suggesting to a patient that they discontinue monotherapy with thiopurines, the most important element is that they agree with this course of action. In fact, very often, the initiative comes from the patient in a context of long-standing clinical, endoscopic and histological remission. It is essential here to consider the patient's risk factors and maintain close follow-up a posteriori, both from a clinical point of view and with FC every three to four months.41–43,79
Thiopurines in Crohn's diseaseA study by Fraser et al., which included 222 patients with CD in remission, assessed discontinuation of AZA. After withdrawal, the proportion of patients remaining in remission at 12, 36 and 60 months was 63%, 44% and 35%, respectively; they found that the length of time taking AZA prior to discontinuation did not affect the rate of relapse once the drug was stopped (p=0.68).73 Another study which included 51 patients with CD treated with AZA 2mg/kg/day for at least six months assessed the effectiveness of maintaining thiopurine treatment (n=27) versus placebo (n=24).80 In a follow-up of up to 12 months, 5% of the patients in the group that continued with AZA had a relapse versus 41% in the placebo group (p≤0.01). Other studies have also confirmed the increased risk of relapse after stopping MP.70 In the study conducted by Kim et al., in those who continued the treatment (n=84), the relapse rate was 29%, 45%, 55% and 61% at 12, 24, 36 and 60 months, respectively. In those who discontinued MP (n=36), the relapse rate was 36%, 71%, 85% and 85% at 12, 24, 36 and 60 months, respectively.81 Bouhnik et al. suggested that thiopurines could be suspended in patients with CD who had been in remission for at least four years.72 The Treton et al. study assessed 66 patients with low-risk CD (steroid-free remission, low CRP, normal white blood cell count, normal haemoglobin, non-smokers) who stopped taking AZA after having been taking it for 68.4 months (range 5.8–85.2 months) and being in clinical remission for an average of 63.6 months. These patients were followed up for 54.5 months (range 20.4–69.6 months). A subgroup of 25 patients who had no associated relapse risk factors (CRP <20mg/l; neutrophil count >4000×109/l; haemoglobin >12g/dl) had a relapse rate of 0% after 18 months of follow-up, and up to 40% after five years. Consequently, the authors propose that in special situations, such as pregnancy or lactation, thiopurines could be withdrawn temporarily in patients with low-risk CD, with a low likelihood of relapse.82 However, it could also be stated that treatment with thiopurines should not be discontinued in these two scenarios. This opinion is based on the fact that the risk of malformations in patients treated with thiopurines is no different from that of the general population, and that the risk of disease reactivation in pregnancy can lead to worse foetal outcomes.83
To sum up, thiopurine therapy in CD should be maintained over a long period of time, yet to be fully defined, before withdrawal is proposed, and, if the patients is a smoker, in view of the negative effects of smoking on the course of the disease, they will have to have stopped prior to any attempt at treatment discontinuation.84 As in UC, although the withdrawal of thiopurines is not recommended, if it is decided to discontinue the therapy, the patient has to be in clinical, endoscopic and histological remission, maintaining clinical follow-up with biomarkers plus endoscopic and imaging assessments where necessary. As mentioned earlier, 5-ASA has not demonstrated effectiveness in maintaining remission in CD, and should not therefore be used after the withdrawal of thiopurines, except in the cases discussed above of mild colonic CD.
MethotrexateMethotrexate is an antagonist of the enzyme folate reductase, which acts on the synthesis of thymidylate and the enzyme thymidylate synthetase. Once inside the cell, methotrexate becomes methotrexate polyglutamate, which reduces cell proliferation, increasing T-cell apoptosis and adenosine concentrations, and altering cytokine production.85 In terms of the route of administration, the oral bioavailability of this drug is variable, reaching approximately 73% of that obtained subcutaneously, although this is not explained purely by the intestinal inflammation characteristic of IBD, as the same situation has also been described in rheumatoid arthritis.86,87 Parenteral administration (intramuscular or subcutaneous) has a quite similar bioavailability and pharmacokinetic profile and is preferred when starting remission maintenance therapy in corticosteroid-dependent CD.88 The usual dose in this scenario is 15–25mg a week. Given its role as a folate antagonist, folic acid supplementation of 1mg daily or 5mg a week is required, and this reduces methotrexate's gastrointestinal and hepatic toxicity.89
Although the role of methotrexate in the maintenance of remission in corticosteroid-dependent CD has been demonstrated in different studies, its role in UC is open to question.90,91 The MERIT-UC study recently conducted by Herfarth et al. assessed 179 patients with active UC (total Mayo Index of 6–12, with a MES ≥2) despite being on previous standard or biological therapy for 48 weeks. They had induction with methotrexate for 16 weeks, and then maintenance for 32 weeks with methotrexate 25mg weekly or placebo. At week 16, the subgroup of 84 patients who were responding to therapy in steroid-free remission were randomised to continue therapy with weekly methotrexate (n=44) or placebo (n=40) until week 48. At the end of the study, 60% of the placebo group (24/40) and 66% of the methotrexate group (29/44) had a relapse of UC, with no statistically significant difference between the two groups. At week 48, 30% of the patients in the placebo group (12/40), and 27% in the methotrexate group (12/44) remained in steroid-free clinical remission.92 As in other previous studies, the authors found that despite the use of methotrexate in UC, there is currently little evidence to support its use in remission maintenance.89,93
Methotrexate is reserved for patients with active CD who are refractory or intolerant to thiopurines.90 In these patients, it has been shown to achieve a remission rate at 40 weeks of 65% (RR: 1.67; 95% CI: 1.05–2.67), with a number needed to treat (NNT) of 4 and remission maintenance of 70–90% at one year, of 59–73% at two years and of 51–52% at three years.91
With regard to withdrawing methotrexate, no studies have specifically assessed patients’ response to this course of action. One retrospective study reviewed 48 patients with CD and 22 with UC who were started on methotrexate (most had previously received AZA, resulting in 35 of them intolerant to thiopurines) for an average of 17.1 months (range 0.5–71 months), with average maintenance dose of 20mg weekly (range 10–25mg); 62 patients started therapy orally and eight by intramuscular injection, all on weekly regimens. They found that 55 patients completed more than three months of therapy, 34 of whom achieved remission (62%). Nineteen patients discontinued therapy, with remission maintenance rates following methotrexate withdrawal of 42%, 21% and 16% at 6, 12 and 18 months, respectively, with only one patient in remission after 18 months.94
Despite the above, the evidence regarding the time to discontinue methotrexate therapy is poor; the studies are in small populations and in general retrospective, which does not allow us to establish very concrete conclusions. The remission criteria used are different in each study, with clinical remission being considered in some cases, but others defining remission as the possibility of remaining steroid-free for three months. However, this is far from the current clinical requirements, where the expectation is to have mucosal and histological healing (defined as the non-existence of acute findings of mucosal activity) as ultimate objective.95 In this context, it is difficult to consider the withdrawal of methotrexate in the absence of clear evidence on how the patients will respond. Once again, therefore, the decision in this scenario should be taken on a case-by-case basis.
There is more evidence on withdrawal of methotrexate in patients with rheumatoid arthritis, where better outcomes have been found with distancing the weekly doses to every two weeks than with stopping the drug completely, with complete discontinuation showing a higher rate of disease reactivation.96
ConclusionWhile seeking to avoid the adverse events of the different therapies in IBD, reduce costs and increase adherence, among other measures, we need to find a way to decrease the number of drugs taken by each patient. In this context, the risk factors associated with a higher relapse rate need to be established. This should allow us to distinguish at an early stage between the low-risk patients in whom the therapy could be reduced and eventually stopped, and those at high risk, in whom discontinuing therapy would only lead to a high relapse rate, with the need for early reintroduction of therapy, and often with the need to scale up to other treatment strategies, including surgery.
To conclude, it is difficult to propose a formula or algorithm for reducing/stopping universal treatment for patients with IBD, as we are always left with the need to make adjustments, assessing each case individually. This means considering primarily the fact that it has to be a joint decision with the patient, having first achieved clinical, endoscopic, histological and radiological remission for a prolonged period.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Sedano Muñoz R, Quera Pino R, Ibanez Lazo P, Figueroa Corona C, Flores Pérez L. Aminosalicilatos, tiopurínicos y metotrexato en la enfermedad inflamatoria intestinal, es posible suspender el tratamiento? Gastroenterol Hepatol. 2019;42:339–347.