Chronic infection with hepatitis C virus is a risk factor for developing atheromatous plaques, although the possible effect of virus clearance is unknown. Our aim was to determine whether or not subclinical atheromatosis improved and there was any modification in the composition of the plaques 12 months after eradication of hepatitis C virus by direct-acting antiviral agents.
Materials and methodsProspective study that included 85 patients with chronic hepatitis C virus infection in different stages of fibrosis who were on direct-acting antiviral agents. Patients with a cardiovascular history, diabetes and kidney disease were excluded. An arterial ultrasound (carotid and femoral) was performed to diagnose atheromatous plaques (defined as intima-media thickness ≥1.5mm) and the composition (percentage of lipids, fibrosis and calcium with HEMODYN4 software) was analysed at the beginning of the study and 12 months after stopping the therapy.
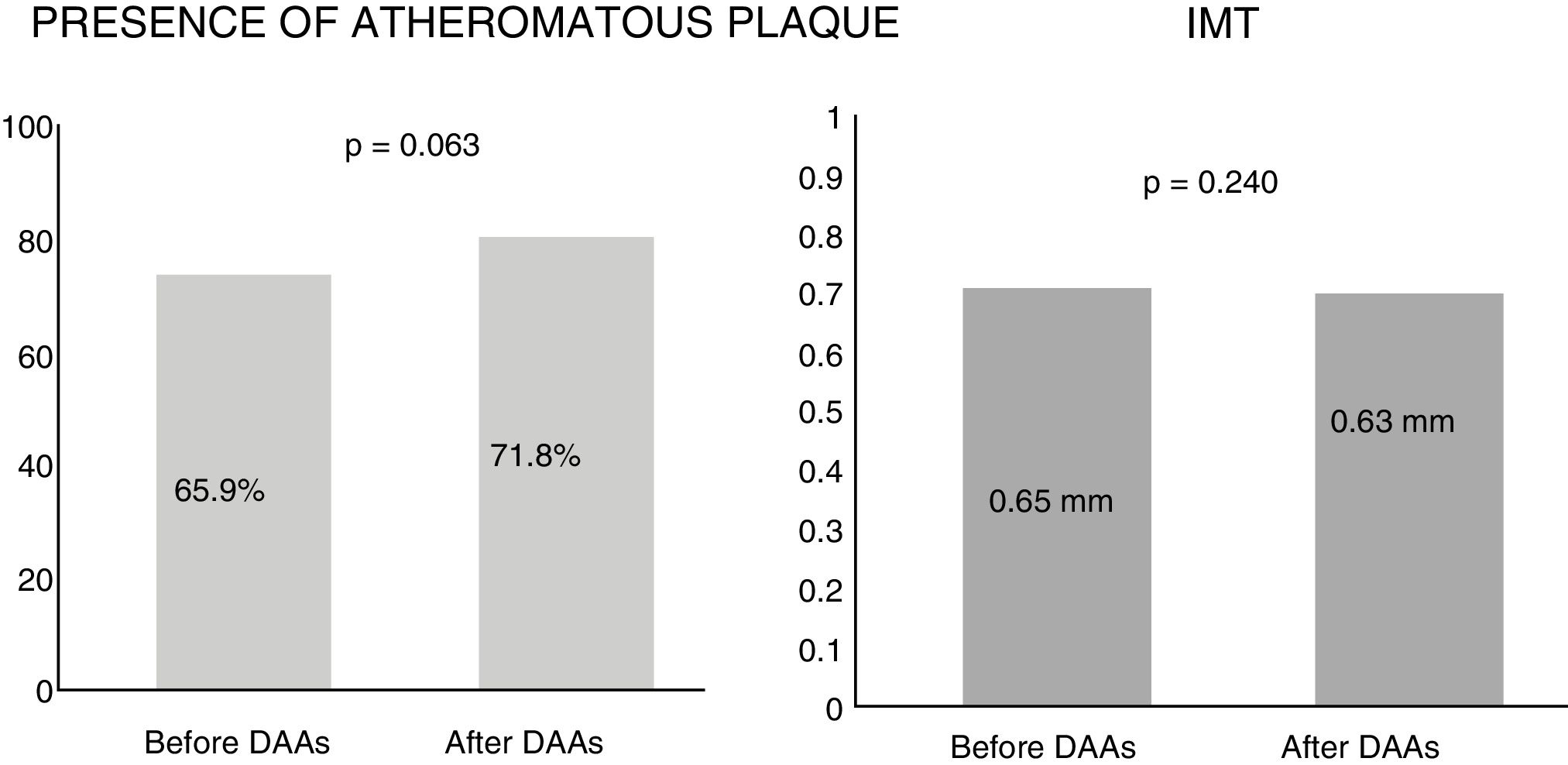
ResultsAfter follow-up no changes were detected in the intima-media thickness (0.65mm vs 0.63mm, p=0.240) or in the presence of plaques (65.9% vs 71.8%, p=0.063). There was also no significant change in their composition or affected vascular territory, with an increase in blood lipid profile (p<0.001) after 12 months of treatment. These results were confirmed in subgroups by severity of liver disease.
DiscussionThe eradication of hepatitis C virus by direct-acting antiviral agents does not improve the atheroma plaques and nor does it vary their composition, regardless of liver fibrosis. More prospective studies are needed to evaluate residual cardiovascular risk after virus eradication.
La infección crónica por el virus de la hepatitis C (VHC) es un factor de riesgo para desarrollar placas de ateroma, aunque se desconoce el posible efecto al eliminar el virus. Nuestro objetivo fue analizar si tras 12 meses de la erradicación del VHC por antivirales de acción directa (AAD) mejoraba la ateromatosis subclínica y existía modificación en la composición de las placas.
Materiales y métodosEstudio prospectivo que incluyó 85 pacientes con infección crónica por VHC en diferentes estadios de fibrosis, sometidos a AAD. Se excluyeron pacientes con antecedentes cardiovasculares, diabetes y enfermedad renal. Se realizó ecografía arterial (carótidas y femorales) para diagnosticar placa de ateroma (definida como grosor íntima-media≥1,5mm) y se analizó su composición (porcentaje de lípidos, fibrosis y calcio con software HEMODYN4) al inicio del estudio y tras 12 meses de finalizar la terapia.
ResultadosTras el seguimiento no se detectaron cambios en el grosor íntima-media (0,65mm vs. 0,63mm, p=0,240) ni en la presencia de placas (65,9% vs. 71,8%, p=0,063). Tampoco hubo modificación significativa en la composición de las mismas ni del territorio vascular afecto, observándose un aumento del perfil lipídico en sangre (p<0,001) tras 12 meses del tratamiento. Estos resultados se confirmaron en subgrupos por gravedad de enfermedad hepática.
DiscusiónLa erradicación del VHC por AAD no mejora las placas de ateroma ni varía su composición, independientemente de la fibrosis hepática. Se precisan más estudios prospectivos que evalúen el riesgo residual cardiovascular tras la erradicación viral.
Hepatitis C virus (HCV) infection continues to be a prevalent disease throughout the world, with an incidence ranging from 1.5% to 2.3% depending on geographical location. The new direct-acting antivirals (DAAs) achieve sustained virologic response (SVR) rates of 95–99%.1 In Spain, since the introduction of the National Strategic Plan for Hepatitis C in 2015, some 100,000 patients have successfully eliminated the virus.2
Chronic HCV infection does not just entail an increased risk of mortality and complications due to liver disease, but also extrahepatic complications.3 Several studies have found that HCV patients exhibit more symptoms associated with immune system dysfunction and metabolic abnormalities than the uninfected population.4
In addition, it has recently been published that HCV infection increases the risk of carotid atherosclerosis, peripheral artery disease, coronary artery disease, cerebrovascular events and cardiovascular mortality.5–7 These data have been analysed in a recent meta-analysis, which supports a possible association between HCV infection and the risk of cardiovascular disease. However, given the heterogeneity of the studies, greater understanding of this association is required.8
Cardiovascular disease is currently one of the leading causes of morbidity and mortality, requiring vascular ultrasound examination to ascertain the cardiovascular risk of each population. The intima-media thickness (IMT) and the presence of plaques in the carotid and femoral arteries are subclinical markers of atherosclerosis and are considered independent predictors of cardiovascular events.9 Another predictor is plaque vulnerability10 measured by the area of the plaque or the degree of stenosis, and the composition or median ultrasound greyscale levels of the plaque. It is accepted that dark and homogeneous plaques indicate the presence of lipids and represent more vulnerable plaques.11
The atherogenic mechanisms of HCV are not fully understood but are believed to be associated with chronic systemic inflammation and the pathogenic effect of the virus on the vessels.12,13 The oxidative stress caused by HCV modifies low-density lipoproteins, causing them to deposit more easily on the arterial walls thereby lowering serum cholesterol levels.14 As a result, these patients develop atherosclerosis without exhibiting the classic pattern of dyslipidaemia.15
These mechanisms, which differ from those of the general population, may modify the composition of atheromatous plaques in HCV patients, although studies are required to confirm this. Various publications concerning the general population have concluded that fibrocalcific plaques are more stable, while lipid plaques are more vulnerable and more often give rise to cardiovascular events.9–11,16–20 It has also been found that lipid plaque buildup can be reversed more easily with intensive lipid-lowering therapy that reduces the lipid core, than fibrocalcific plaques.21
The use of safe and effective DAAs to cure HCV raises the question of whether elimination of the virus might also improve cardiovascular manifestations. Most of the data currently available derives from patients treated with interferon (IFN)-based therapies and suggest that sustained virologic response reduces cardiovascular risk.22–25 However, due to the design of these studies and bias in the results, it cannot be ascertained whether this effect is due to elimination of the virus or to the use of IFN itself.
We conducted this study precisely in order to answer this question. We assessed the impact of HCV on atheromatous plaques in both the carotid and femoral arteries after 12 months of SVR in a cohort of patients with chronic hepatitis C treated with DAAs. The effect was stratified by severity of liver disease and the various cardiovascular risk factors. We also analysed the composition of the plaques detected by ultrasound greyscale and assessed any changes to the percentage of lipids, fibrosis and calcium after virus elimination.
Patients and methodsThis was a prospective study conducted in two hospitals in Lleida (Hospital Universitario Arnau de Vilanova and Hospital Universitario de Santa María), in collaboration with the Unit for the Detection and Treatment of Atherothrombotic Disease (UDETMA) of the Hospital Universitario Arnau de Vilanova, from 2015 to 2018.
Initially, 185 patients with chronic HCV infection who met none of the exclusion criteria for the study (listed below) were included. These patients were treated with direct-acting antivirals according to the protocols of the European Association for the Study of the Liver26 and the Asociación Española para el Estudio del Hígado [Spanish Association for the Study of the Liver],27 as well as the HCV treatment guidelines of the Institut Català de Salut [Catalan Health Institute]28 that were valid during the study period (2015–2018). After the follow-up period (12 months after virus elimination with treatment), only 85 patients (45% of the initial sample) underwent the second vascular ultrasound. Despite this loss to follow-up, patient characteristics in terms of CV risk factors, viral genotype and severity of liver disease changed very little. Chronic HCV infection was confirmed by anti-HCV antibodies and viral RNA (HCV-RNA: reverse transcription polymerase chain reaction with limit of detection of 12IU/ml) of any genotype and degree of hepatic fibrosis. The HCV-RNA tests were repeated after four weeks of treatment, at the end of treatment and 12 weeks after completion. SVR was defined as undetectable HCV-RNA 12 weeks after stopping DAA therapy.
The exclusion criteria were: (1) age <30 or >75 years; (2) decompensated cirrhosis; (3) hepatocellular carcinoma or other tumours; (4) non-HCV chronic liver disease (alcoholic, hepatitis B virus, autoimmune disease or iron overload); (5) HIV infection; (6) acute or chronic inflammatory or infectious disease; (7) prior history of cardiovascular events according to the International Classification of Diseases, 10th revision (ICD10-CM): —(a) cerebrovascular disease: transient ischaemic attack and or stable stroke; (b) coronary heart disease: acute myocardial infarction, unstable angina pectoris, arrhythmia and/or congestive heart failure; and (c) peripheral artery disease of the lower limbs or aortic aneurysm—; (8) chronic kidney failure (CKD-EPI equation<60ml/min/1.73m2); (9) diagnosis of diabetes mellitus; (10) patients with dyslipidaemia treated with lipid-lowering drugs; and (11) history of treated hypertension.
The study was evaluated and approved by the ethics committee of the participating hospitals and all patients signed the study's informed consent form.
Clinical and laboratory dataThe patients’ clinical and laboratory data were collected during the hepatology visit prior to treatment and 12 months after completing the direct-acting antiviral therapy.
Fasting blood samples were used to measure the following serum parameters: glucose, total cholesterol (TC), LDL and HDL cholesterol, triglycerides (TG), creatinine and liver transaminases (AST and ALT). The lipid coefficients were calculated: non-HDL cholesterol (total cholesterol – HDL cholesterol), TG/HDL cholesterol (indicator of insulin resistance) and TC/HDL cholesterol (atherogenic index). All the tests were conducted before and after treatment in the same laboratory. HCV-RNA (RT-PCR: limit 12IU/ml) and the virus genotype (INNO-liPA HCV II, Bayer®) were detected. All patients underwent a transient elastography (FibroScan®, Echosens, Paris, France) to quantify the degree of hepatic fibrosis, classifying it as per the manufacturer's recommendations (less than 7.5kPa — no fibrosis [F0-1], 7.6–9.5kPa, moderate fibrosis [F2], 9.6–12.5kPa — significant fibrosis (F3) and more than 12.5kPa liver cirrhosis [F4]). Transaminase levels were tested before and after treatment to assess the presence of hepatic steatosis, using the Hepatic Steatosis Index (HSI=8×ALT/AST+BMI+2 [if diabetes mellitus]+2 [if female] >36).
The anthropometric data to calculate the body mass index (BMI) and the hypertension diagnosis were collected at the Unit for the Detection and Treatment of Atherothrombotic Disease before treatment and after 12 weeks of therapy and at the vascular Doppler ultrasound. Weight (kilograms) and height (centimetres) were measured and patients were classified by BMI (normal weight: BMI=18.5–24.9kg/m2; overweight: BMI=25–29.9 and obese: BMI>30). To assess hypertension (systolic blood pressure>135mmHg and/or diastolic blood pressure>85mmHg), blood pressure was taken three times at least 30min apart using an arm sphygmomanometer. The smoking history of all patients was investigated, classifying them as smokers (quantifying the number of cigarettes smoked per day), former smokers or non-smokers, and evaluating the classic risk factors of atherosclerotic disease.
Evaluation of carotid and femoral atherosclerosisSubclinical atherosclerosis was defined as increased IMT (IMT>1.5mm) and the finding of one or more atheromatous plaques at any of the sites studied.
Subclinical atherosclerosis was analysed by two technical experts of the Unit for the Detection and Treatment of Atherothrombotic Disease with a General Electric, model vivid-i ultrasound system with a 12L-RS vascular probe, following a standardised protocol with an interobserver Kappa coefficient of 1. The images were analysed blind by a single reader from the Unit.29 Two ultrasound studies were performed: at study enrolment (prior to DAA therapy) and 12 months after achieving SVR.
Atherosclerotic burden was evaluated in different vascular territories: the carotid arteries (common carotid, carotid bulb and internal carotid bilaterally: right and left) and the femoral arteries (common and superficial, also bilaterally). The IMT and presence of plaques (defined as the difference in lumen of the intima and the adventitia of the arterial wall of at least 1.5mm [IMT>1.5mm]) was analysed in each of these 10 segments by high-resolution B-mode colour Doppler ultrasound in accordance with the Mannheim consensus.30 The IMT was measured in the distal wall of all the aforementioned vascular territories, as long as no atheromatous plaques were present. The IMT value was the mean value of all the values. Both increased IMT as well as the finding of carotid and femoral artery plaques in asymptomatic patients are independent predictive factors of cardiovascular disease.19 Another ultrasound marker of cardiovascular events is atheromatous plaque vulnerability, assessed by measuring the area and the composition by greyscale according to echogenicity.16
The plaques were measured in all examined areas, with the total plaque area being the sum of all the values obtained (carotid and femoral plaques). The number of pixels within the outline of the plaque was used to estimate the size in that ultrasound image (140pixels/cm).
Software (HEMODYN 4M, Dinap SRL, Argentina) was used in the atheromatous plaque composition study to calculate the percentage of lipids, fibrous tissue and calcium in the different plaques examined. This was based on a computerised greyscale median analysis according to echogenicity, which mapped the pixels of the plaque and produced more quantitative, objective and less observer-dependent results.16 This characterisation has been shown to have a strong correlation with the histological components of the atheromatous plaque.17,18
Statistical analysisA descriptive analysis was performed that included frequencies and percentages for the qualitative variables, which were compared with Pearson's chi-square test, while means and standard deviations were applied to the quantitative variables. A logistic regression with clinical and clinical chemistry parameters was performed to assess the correlation between atheromatous plaques before and after treatment on the entire cohort of HCV patients. McNemar's test was used to assess changes to the atheromatous plaques (IMT>1.5mm, dichotomous variable) before and after antiviral therapy. To assess changes in IMT (only measured in patients without atheromatous plaque IMT>1.5mm, quantitative) and in the various laboratory parameters, Student's t-test was used for paired data in the variables that followed a normal distribution, and the Wilcoxon test in variables with non-normal distribution. Assessment of the changes was segmented in accordance with the degree of fibrosis and the different cardiovascular risk factors. Statistical significance was set at a p-value <0.05. All analyses were performed using the software SPSS (version 24).
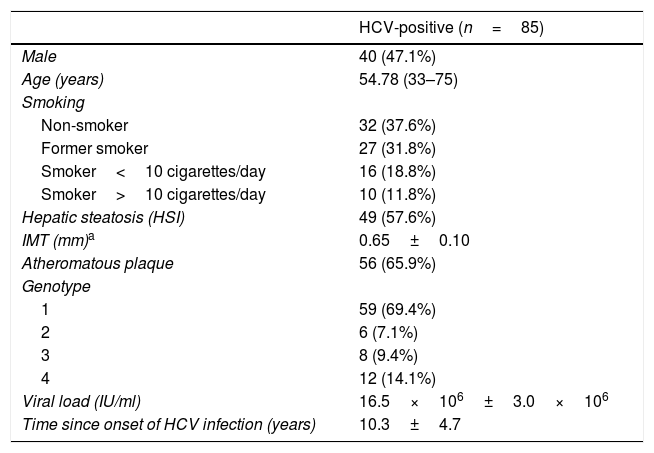
ResultsCharacteristics of hepatitis C virus patients before treatment with direct-acting antiviralsThe characteristics of the 85 patients studied are summarised in Table 1. 47% were men and the average age was 55. Only 16.5% of the patients were over the age of 65. Upon assessment of the classic atherosclerotic disease risk factors, it was found that 25.9% were obese (BMI>30kg/m2), 14.1% were hypertensive and 3.5% had dyslipidaemia. None of the patients had diabetes mellitus. 62.4% were smokers. 57.6% of the sample had hepatic steatosis according to the HSI. The mean blood pressure, glucose, creatinine, lipid profile and insulin resistance index (measured by the TG/HDL ratio) values were within normal range. Most patients were infected by genotype 1. 57.5% of patients had advanced chronic liver disease (F3–F4), 34% of which also had cirrhosis.
Characteristics of HCV patients treated with direct-acting antivirals (DAAs).
| HCV-positive (n=85) | |
|---|---|
| Male | 40 (47.1%) |
| Age (years) | 54.78 (33–75) |
| Smoking | |
| Non-smoker | 32 (37.6%) |
| Former smoker | 27 (31.8%) |
| Smoker<10 cigarettes/day | 16 (18.8%) |
| Smoker>10 cigarettes/day | 10 (11.8%) |
| Hepatic steatosis (HSI) | 49 (57.6%) |
| IMT (mm)a | 0.65±0.10 |
| Atheromatous plaque | 56 (65.9%) |
| Genotype | |
| 1 | 59 (69.4%) |
| 2 | 6 (7.1%) |
| 3 | 8 (9.4%) |
| 4 | 12 (14.1%) |
| Viral load (IU/ml) | 16.5×106±3.0×106 |
| Time since onset of HCV infection (years) | 10.3±4.7 |
Quantitative variable: mean±SD (range); qualitative variable: n (%).
HCV: chronic hepatitis C virus; HSI: hepatic steatosis index; IMT: intima-media thickness.
At the time of recruitment, 61.2% of the patients were naive and 38.8% were non-responsive to combination therapy (pegylated IFN and ribavirin). All were treated with DAAs in accordance with the HCV treatment guidelines as per the criteria proposed by the Catalan Health Institute from 2015 to 2018: 51.6% with sofosbuvir/ledipasvir±ribavirin for 12–24 weeks, 17.6% with sofosbuvir–simeprevir±ribavirin for 12 weeks, 4.7% with sofosbuvir±ribavirin for 24 weeks, 7.1% with sofosbuvir–daclatasvir±ribavirin for 12 weeks and 19% with ombitasvir–paritaprevir–ritonavir and dasabuvir±ribavirin for 12–24 weeks. 8.2% failed to respond to first-generation protease inhibitors (boceprevir/telaprevir) and were subsequently treated with second-generation protease inhibitors, achieving sustained virologic response.
Subclinical atherosclerosis and risk factors at the start of the studyNo atheromatous plaques were observed in 29 patients (34.1%), finding a mean IMT of 0.65±0.10mm. In contrast, plaques in one or both vascular territories (carotid or femoral) were detected in 56 patients (65.9%). None of the study participants had clinically significant vascular stenosis (defined as >60% narrowing of the vascular lumen due to atheromatous plaque) or plaque ulceration.
The male gender, a prior history of smoking, genotype, fibrosis, body mass index, systolic blood pressure, HDL cholesterol and triglyceride levels and the insulin resistance index were all found to be significantly associated with the presence of atheromatous plaque. In the multivariate regression model, only gender (OR: 6.02 [CI: 1.46–24.78]; p=0.013) and the viral genotype (OR: 2.56 [CI: 1.13–5.77]; p=0.024) were independent factors of plaque in HCV patients.
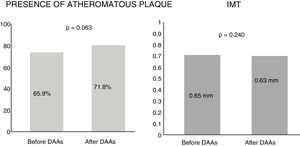
Subclinical atherosclerosis after antiviral therapyBy analysing the mean IMT after 12 months of antiviral therapy, an improving trend was observed, but this was not statistically significant (0.65mm vs 0.63mm). Similarly, no significant differences were found in the presence of atheromatous plaques after one year of treatment (65.9% vs 71.8%, p=0.063) (Fig. 1). The IMT prior to antiviral therapy was analysed in 29 patients (who did not exhibit plaques) but only in 24 patients after treatment during follow-up, as more atheromatous plaques were observed.
In terms of liver disease parameters, a fall in transaminase levels was observed, both AST (56.69IU/ml vs 25.18IU/ml, p<0.001) and ALT (57.42IU/ml vs 19.18IU/ml, p<0.001), as well as the presence of hepatic steatosis calculated using the HSI (57.6% prior to DAAs vs 38.8% after DAAs, p=0.002). Improved hepatic fibrosis measured by liver elastography (FibroScan®) was also observed after 12 months of treatment (F1=18.8%, F2=23.5%, F3=23.5%, F4=34.1% vs F1=40%, F2=20%, F3=14.1%, F4=25.9%; p<0.001).
No changes in BMI, systolic blood pressure, diastolic blood pressure or blood glucose levels were found. A significant worsening in the lipid profile after 12 months of antiviral therapy was observed (total cholesterol 172.12mg/dl vs 199.65mg/dl, p<0.001; non-HDL cholesterol 118.63mg/dl vs 146.25mg/dl, p<0.001, and LDL cholesterol 95.10mg/dl vs 111.97mg/dl, p<0.001), with a significant increase in the atherogenic index (TC/HDL) detected in the first year in patients who had received antiviral therapy (2.23±1.56 vs 3.39±0.99; p<0.001). No changes in HDL cholesterol or triglyceride levels, or in the insulin resistance index (TG/HDL) were found (Table 2).
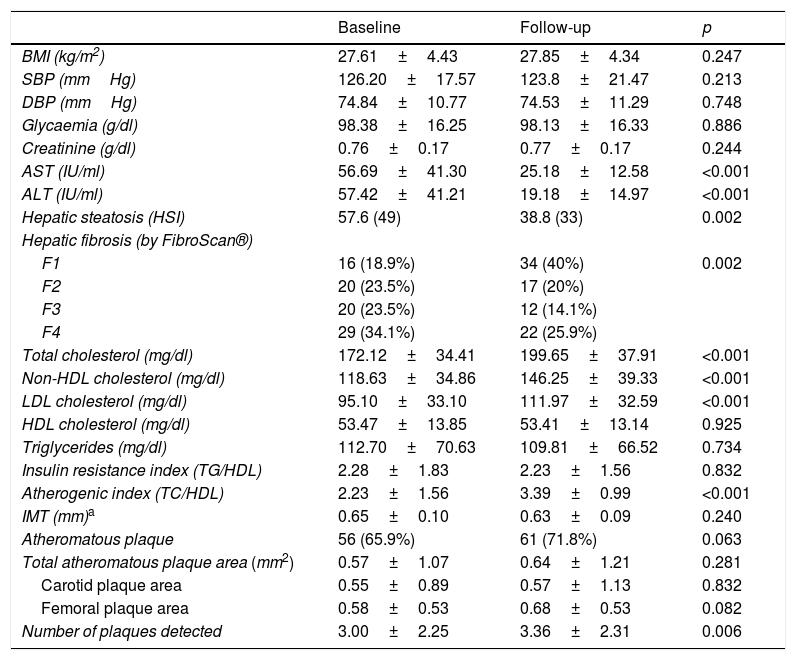
Characteristics of the HCV patients before and after 12 months of treatment with direct-acting antivirals (DAAs).
| Baseline | Follow-up | p | |
|---|---|---|---|
| BMI (kg/m2) | 27.61±4.43 | 27.85±4.34 | 0.247 |
| SBP (mmHg) | 126.20±17.57 | 123.8±21.47 | 0.213 |
| DBP (mmHg) | 74.84±10.77 | 74.53±11.29 | 0.748 |
| Glycaemia (g/dl) | 98.38±16.25 | 98.13±16.33 | 0.886 |
| Creatinine (g/dl) | 0.76±0.17 | 0.77±0.17 | 0.244 |
| AST (IU/ml) | 56.69±41.30 | 25.18±12.58 | <0.001 |
| ALT (IU/ml) | 57.42±41.21 | 19.18±14.97 | <0.001 |
| Hepatic steatosis (HSI) | 57.6 (49) | 38.8 (33) | 0.002 |
| Hepatic fibrosis (by FibroScan®) | |||
| F1 | 16 (18.9%) | 34 (40%) | 0.002 |
| F2 | 20 (23.5%) | 17 (20%) | |
| F3 | 20 (23.5%) | 12 (14.1%) | |
| F4 | 29 (34.1%) | 22 (25.9%) | |
| Total cholesterol (mg/dl) | 172.12±34.41 | 199.65±37.91 | <0.001 |
| Non-HDL cholesterol (mg/dl) | 118.63±34.86 | 146.25±39.33 | <0.001 |
| LDL cholesterol (mg/dl) | 95.10±33.10 | 111.97±32.59 | <0.001 |
| HDL cholesterol (mg/dl) | 53.47±13.85 | 53.41±13.14 | 0.925 |
| Triglycerides (mg/dl) | 112.70±70.63 | 109.81±66.52 | 0.734 |
| Insulin resistance index (TG/HDL) | 2.28±1.83 | 2.23±1.56 | 0.832 |
| Atherogenic index (TC/HDL) | 2.23±1.56 | 3.39±0.99 | <0.001 |
| IMT (mm)a | 0.65±0.10 | 0.63±0.09 | 0.240 |
| Atheromatous plaque | 56 (65.9%) | 61 (71.8%) | 0.063 |
| Total atheromatous plaque area (mm2) | 0.57±1.07 | 0.64±1.21 | 0.281 |
| Carotid plaque area | 0.55±0.89 | 0.57±1.13 | 0.832 |
| Femoral plaque area | 0.58±0.53 | 0.68±0.53 | 0.082 |
| Number of plaques detected | 3.00±2.25 | 3.36±2.31 | 0.006 |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; BMI: body mass index; DBP: diastolic blood pressure; HDL: high-density lipoprotein; HSI: Hepatic Steatosis Index; IMT: carotid intima-media thickness; LDL: low-density lipoprotein; SBP: systolic blood pressure; TC: total cholesterol; TG: triglycerides.
After 12 months of treatment with DAAs, there were also no differences found in the total area of plaques detected (p=0.281) in either the carotid or femoral arteries, but there were more plaques detected during this period of time (p=0.006).
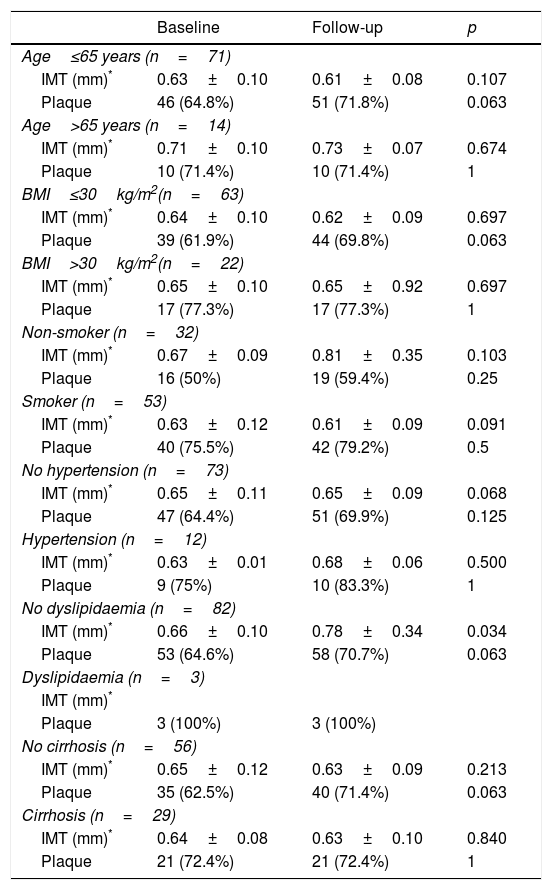
Having observed the factors associated with changes to the IMT and the presence of plaque after treatment, none of the baseline parameters or changes in clinical chemistry was associated (p>0.10). In line with these results, the lack of change to IMT or the presence of atheromatous plaque was confirmed in the patient subsets categorised by cardiovascular risk factors and liver disease severity (Table 3). A trend towards IMT improvement was detected in patients under the age of 65 who were not obese, non-smokers, non-hypertensive and had no dyslipidaemia, in both cirrhotic and non-cirrhotic patients, but this was not statistically significant. However, the strength of this analysis is limited by the small number of patients in each subset.
Changes in IMT and presence of atheromatous plaque before and after 12 months of treatment with DAAs by cardiovascular risk factor subsets.
| Baseline | Follow-up | p | |
|---|---|---|---|
| Age≤65 years (n=71) | |||
| IMT (mm)* | 0.63±0.10 | 0.61±0.08 | 0.107 |
| Plaque | 46 (64.8%) | 51 (71.8%) | 0.063 |
| Age>65 years (n=14) | |||
| IMT (mm)* | 0.71±0.10 | 0.73±0.07 | 0.674 |
| Plaque | 10 (71.4%) | 10 (71.4%) | 1 |
| BMI≤30kg/m2(n=63) | |||
| IMT (mm)* | 0.64±0.10 | 0.62±0.09 | 0.697 |
| Plaque | 39 (61.9%) | 44 (69.8%) | 0.063 |
| BMI>30kg/m2(n=22) | |||
| IMT (mm)* | 0.65±0.10 | 0.65±0.92 | 0.697 |
| Plaque | 17 (77.3%) | 17 (77.3%) | 1 |
| Non-smoker (n=32) | |||
| IMT (mm)* | 0.67±0.09 | 0.81±0.35 | 0.103 |
| Plaque | 16 (50%) | 19 (59.4%) | 0.25 |
| Smoker (n=53) | |||
| IMT (mm)* | 0.63±0.12 | 0.61±0.09 | 0.091 |
| Plaque | 40 (75.5%) | 42 (79.2%) | 0.5 |
| No hypertension (n=73) | |||
| IMT (mm)* | 0.65±0.11 | 0.65±0.09 | 0.068 |
| Plaque | 47 (64.4%) | 51 (69.9%) | 0.125 |
| Hypertension (n=12) | |||
| IMT (mm)* | 0.63±0.01 | 0.68±0.06 | 0.500 |
| Plaque | 9 (75%) | 10 (83.3%) | 1 |
| No dyslipidaemia (n=82) | |||
| IMT (mm)* | 0.66±0.10 | 0.78±0.34 | 0.034 |
| Plaque | 53 (64.6%) | 58 (70.7%) | 0.063 |
| Dyslipidaemia (n=3) | |||
| IMT (mm)* | |||
| Plaque | 3 (100%) | 3 (100%) | |
| No cirrhosis (n=56) | |||
| IMT (mm)* | 0.65±0.12 | 0.63±0.09 | 0.213 |
| Plaque | 35 (62.5%) | 40 (71.4%) | 0.063 |
| Cirrhosis (n=29) | |||
| IMT (mm)* | 0.64±0.08 | 0.63±0.10 | 0.840 |
| Plaque | 21 (72.4%) | 21 (72.4%) | 1 |
BMI: body mass index; IMT: carotid intima-media thickness.
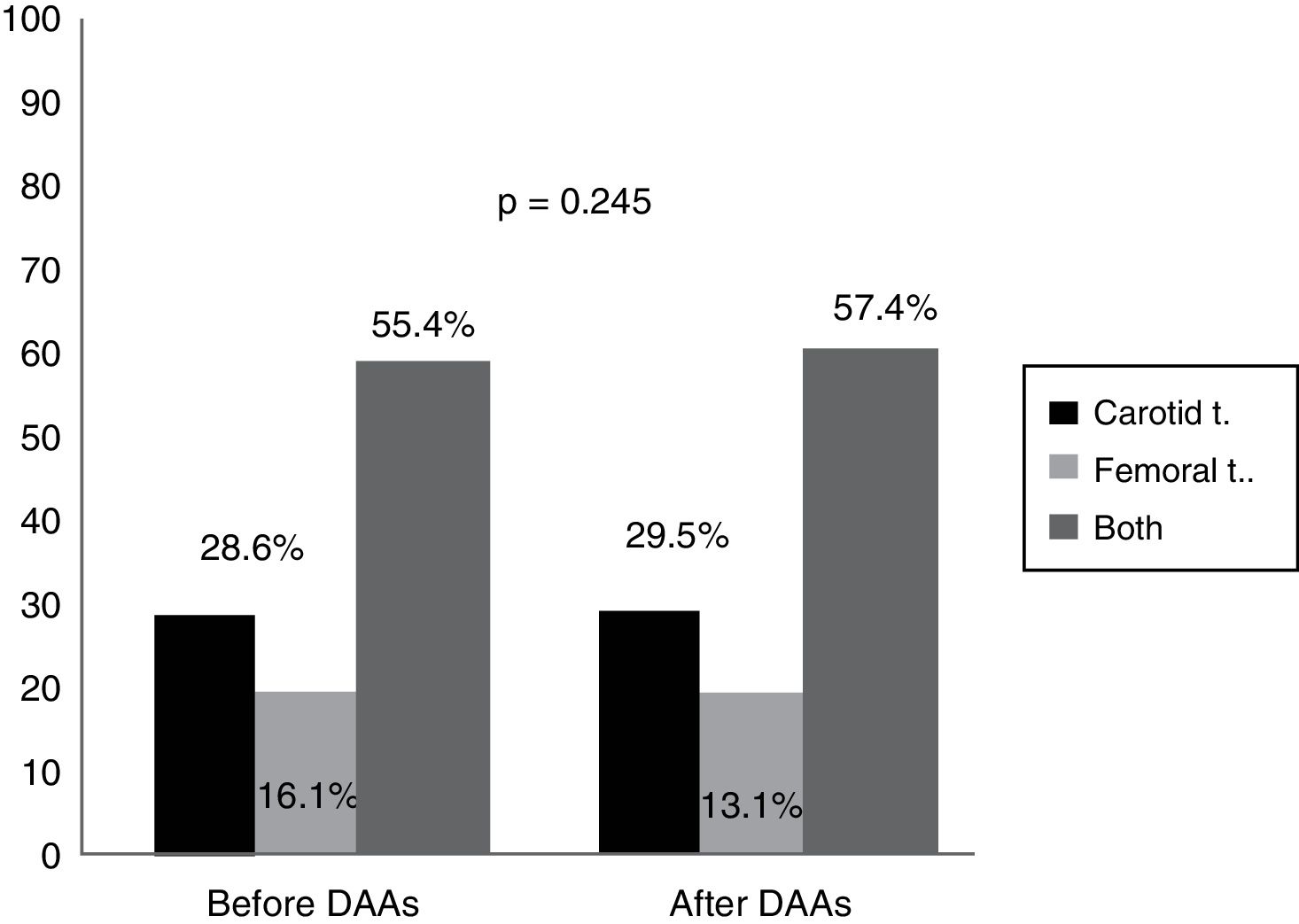
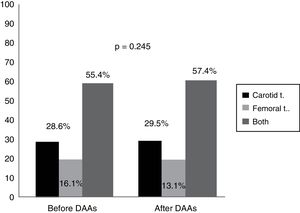
An analysis of the vascular distribution of the plaques did not reveal any significant differences either in the carotid or femoral arteries or in both vascular territories prior to treatment or 12 months after completion of therapy (p=0.245) (Fig. 2).
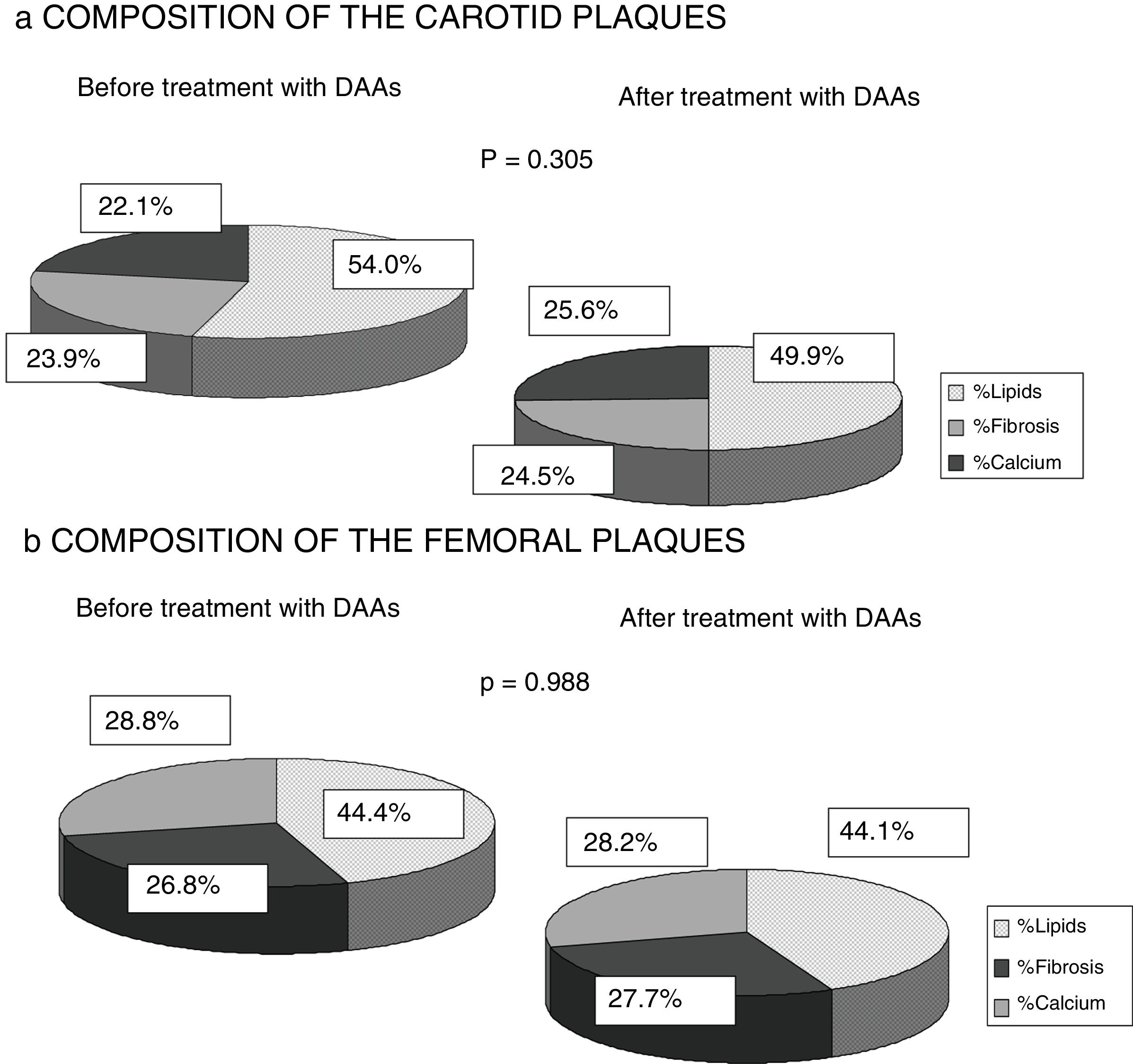
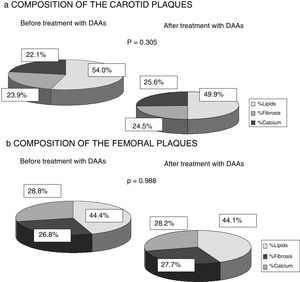
Finally, any changes to the composition of the atheromatous plaques measured by ultrasound greyscale before treatment and 12 months after antiviral therapy were assessed. No significant changes were identified in the percentage of lipids, fibrosis or calcium of the plaques found in either the carotid or femoral territories (p=0.305; p=0.988). A trend towards a falling percentage of lipids in carotid plaques (prior to DAAs 54.1% vs 49.9% after 12 months of treatment) and a higher percentage of fibrosis and calcium (23.9% vs 24.5% and 22.1% vs 25.6%) was observed, while no percentage changes were found in femoral artery plaques (Fig. 3).
DiscussionOur study did not identify changes to IMT or the presence of atheromatous plaques in either vascular territory studied (carotid and femoral) after 12 months of treatment with direct-acting antivirals in chronic HCV patients. As Fig. 1 shows, there was a slight trend towards IMT improvement and a 6% increase in atheromatous plaques, but this was not significant (p=0.063). These differences were not seen in the patient sample as a whole, nor when subdividing the patients by cardiovascular risk factors and liver disease severity (Table 3).
These results are not consistent with other studies that show that virus elimination, whether spontaneous or, more typically, achieved with IFN-based treatment regimens,22–25 can improve cardiovascular outcomes, although it is not clear whether the positive effect observed is due to elimination of the virus, to IFN itself or to selection bias, as they are highly selected populations.
Our data quantify the amount of plaque in the carotid territory as well as in the femoral territory, which has not been done before in other studies. Although no significant differences were observed, as Fig. 2 shows, plaque distribution by territory after treatment increased slightly in both the carotid and femoral territories (from 55.4% prior to treatment to 57.4% after treatment, p=0.245). This extension of the ultrasound examination in population studies, both in carotid as well as femoral vessels, increases the likelihood of detecting atherosclerosis.29
This study found that virus elimination causes levels of total cholesterol, LDL cholesterol and non-HDL cholesterol to increase (described as more atherogenic particles) after 12 months of antiviral therapy. These data are consistent with findings published in patients treated with IFN21 and also highlight the influence of the virus on lipid metabolism.11–14 No significant differences in blood glucose levels or the insulin resistance index measured by the TG/HDL ratio were identified (Table 2). A significant improvement in transaminase levels, hepatic steatosis measured by the Hepatic Steatosis Index and liver stiffness measured by liver elastography were observed, confirming the findings of various studies that virus elimination is not synonymous with curing the infection, but rather that it improves liver disease in the short- and medium-term.22
Our study involved the only Spanish cohort of chronic HCV-monoinfected patients 12 months after virus elimination with DAAs. It suffers from the limitations intrinsic to a longitudinal or follow-up design as many patients were lost to follow-up and did not attend the ultrasound scan after 12 months of therapy, resulting in a small sample size (of the 185 patients who began the study, only 85 completed it). These losses can be explained by the geographical distribution of our province; patients from the peripheral areas found it difficult to reach the hospital due to the remoteness of their towns, choosing not to attend after HCV elimination. There was also selection bias as patients with diabetes mellitus at the time of the study were excluded to avoid confounding factors that could predispose patients to the development of atheromatous plaques. Previous studies have shown greater glucose metabolism control24 in these patients, which we were unable to assess for the reason given above.
One strength of our research is the fact that it is the first study to analyse the composition of atheromatous plaques by ultrasound greyscale. It was found that infected patients exhibited plaques with a higher lipid percentage (carotid plaques 54.6% and femoral plaques 44.4%). This finding could be explained by the influence of HCV on lipid metabolism, as some studies have shown that the virus binds to LDL particles for transport in the blood, changing them and facilitating their deposit on the vascular endothelium.12–14 As Fig. 3 shows, there were also no significant differences found 12 months after HCV elimination with DAAs. However, the carotid plaque lipid content did fall (54.55% prior to treatment vs 50.96% after treatment), while the percentage of fibrosis and calcium increased, albeit not to a statistically significant extent. This finding may be of interest as population studies have shown that less-fatty plaques are more stable and give rise to fewer cardiovascular events.15,18 Published studies of HCV patients treated with IFN22–25 report lower cardiovascular mortality, possibly because the plaques are more organised and less vulnerable rather than due to a fall in the number of atheromatous plaques, although this has not been analysed in any publication to date.
In conclusion, data about the effect that the new antiviral therapies could have on the presence and composition of atheromatous plaques are scarce. Our results suggest that this effect may be minimal, as no change in IMT or the quantity of plaques in the carotid and/or femoral territories were seen 12 months after HCV elimination with DAAs. However, it is important to recognise that this was a small sample and it is not known whether there may be longer-term benefits. These data may support the need for routine non-invasive cardiovascular tests, like vascular ultrasound, which would improve the risk stratification in this population, even after virus elimination. There is also a lack of data about lipid-lowering therapy in these patients to facilitate plaque regression and therefore improve the long-term cardiovascular prognosis. As such, more prospective studies that evaluate the residual cardiovascular risk after virus elimination are required.
Conflicts of interestThe authors declare that they have no conflicts of interest regarding the content of this manuscript.
The authors would like to thank the entire team of the Unit for the Detection and Treatment of Atherothrombotic Disease for conducting the vascular ultrasounds for the detection of subclinical atherosclerosis.
Please cite this article as: Revuelto Artigas T, Betriu Bars À, Zaragoza Velasco N, Gómez Arbones X, Vidal Ballester T, Piñol Felis C, et al. El tratamiento antiviral no mejora la ateromatosis subclínica en pacientes con hepatitis crónica por virus de la hepatitis C. Gastroenterol Hepatol. 2019;42:362–371.