Autologous haematopoietic stem cell transplantation (AHSCT) is an accepted treatment in refractory Crohn's disease (CD).
Material and methodsData on patients with refractory CD subjected to AHSCT are collected at the Hospital Universitario Ramón y Cajal in Madrid and the results obtained are described retrospectively.
ResultsSeven patients in total have received AHSCT due to refractory CD in our centre. Three patients (43%) presented with clinical and endoscopic remission; one patient (14%) clinical improvement without remission and three patients (43%) remained active with the need to restart treatment in the assessment of the initial response to the AHSCT (after six months). Symptoms recurred in five of the seven patients (71%) and all of them had to restart medical treatment after an average of 13.8 months (range: 3–30 months). Only one patient needed surgery after the AHSCT. At the end of the follow-up, after a mean of 48 months (range: 17–78 months), 5/7 (71%) of the patients were in clinical remission with or without treatment.
ConclusionAHSCT may be a promising therapeutic option for patients with refractory CD. Its usefulness lies in the fact that it can produce clinical remission without treatment in some patients, but also that it can make the disease treatable, obtaining a response to certain treatments in patients who had previously lost it.
El trasplante autólogo de precursores hematopoyéticos (TPHA) es una modalidad de tratamiento aceptada para la enfermedad de Crohn (EC) refractaria.
Material y métodosSe recogen los pacientes con EC refractaria sometidos a TPHA en el hospital Universitario Ramón y Cajal de Madrid y se describen de forma retrospectiva los resultados obtenidos.
ResultadosUn total de 7 pacientes han recibido TPHA debido a EC refractaria en nuestro centro. Tres pacientes (43%) presentaron remisión clínica y endoscópica; un paciente (14%) mejoría clínica sin remisión y 3 pacientes (43%) permanecían activos con necesidad de reinicio del tratamiento en la valoración de la respuesta inicial al TPHA (a 6 meses). Los síntomas recurrieron en 5 de los 7 pacientes (71%), y todos ellos requirieron reinicio de tratamiento médico a una media de 13,8 meses (rango: 3-30 meses). Solo un paciente requirió cirugía tras el TPHA. Al final del seguimiento a una media de 48 meses (rango: 17-78 meses) 5/7 (71%) de los pacientes estaban en remisión clínica con o sin tratamiento.
ConclusiónEl TPHA puede ser una opción terapéutica prometedora para pacientes con EC refractaria. Su utilidad radica en que puede producir la remisión clínica sin tratamiento en algunos pacientes, pero también en que puede hacer la enfermedad tratable, consiguiendo respuesta a determinados tratamientos en pacientes que la habían perdido previamente.
Crohn's disease (CD) is an immunologically mediated chronic disease characterised by episodic intestinal inflammation.1 In spite of the great advances and the multiple therapeutic options currently available for CD, 25% of patients are refractory and over 50% have a lack of response to available treatments.2,3
Haematopoietic stem cell transplantation (HSCT) consists of the administration of intensive chemotherapy followed by an intravenous infusion of haematopoietic stem cells. Autologous HSCT (AHSCT) is the accepted modality for refractory autoinflammatory diseases, including refractory CD, due to the high mortality rate associated with allogeneic HSCT which is not acceptable for non-cancerous diseases.4 Although its mechanism of action is not fully understood, it is based on producing an ablation of the patient's autoreactive T lymphocytes through the administration of chemotherapy agents, and on the generation of a new repertoire of tolerant T lymphocytes thanks to the implantation of stem cells.5,6
The current evidence of the use of AHSCT in refractory CD is based mainly on case series with apparent clinical benefits and on a single randomised trial (Autologous Stem Cell Transplant In Crohn's Disease [ASTIC]7). The first reviews to be found on this subject in the literature include cases of patients with CD who showed improvement in their CD symptoms after receiving HSCT for another disease.8,9 Later, there were a number of short series of patients who received AHSCT specifically for their refractory CD,10–12 among which was the Chicago series published by Burt et al.10 The only randomised trial in this field (ASTIC) was published in 2015.7 The ASTIC trial included patients with active refractory CD and all were subjected to the mobilisation phase. They were subsequently randomised to receive early (n=23) or late AHSCT (control group that continued with conventional treatment and received the AHSCT at one year, n=22) in order to determine whether or not the therapy received in the mobilisation phase could induce remission alone. The primary endpoint was to achieve clinical remission (Crohn's Disease Activity Index [CDAI]<150) with endoscopic mucosal healing at one year, with no active treatment in the last three months. The trial showed no benefit in the primary endpoint from the AHSCT compared to conventional therapy. However, it has been widely suggested that the trial's primary endpoint was too strict; it was stricter than is usually required in new drug trials. Nonetheless, there was reported benefit in some of the secondary endpoints, such as improvement in clinical activity (CDAI) and endoscopic activity (Simple Endoscopic Score for Crohn's Disease), as well as in quality of life.7 Subsequently, two non-randomised trials13,14 reported benefits in relation to achieving clinical and endoscopic remission without treatment after the AHSCT (61% at one year13), but also in relation to responding to treatments to which they had previously lost response (57%14 and 80%14 of the re-treated patients obtained a response).
The aim of this retrospective study was to describe both clinical benefits and adverse effects in patients with refractory CD who had AHSCT at our centre.
Patients and methodsWe conducted a retrospective search for patients who received AHSCT for refractory CD at Hospital Universitario Ramón y Cajal in Madrid from June 2011 to December 2017. We report on the results obtained below.
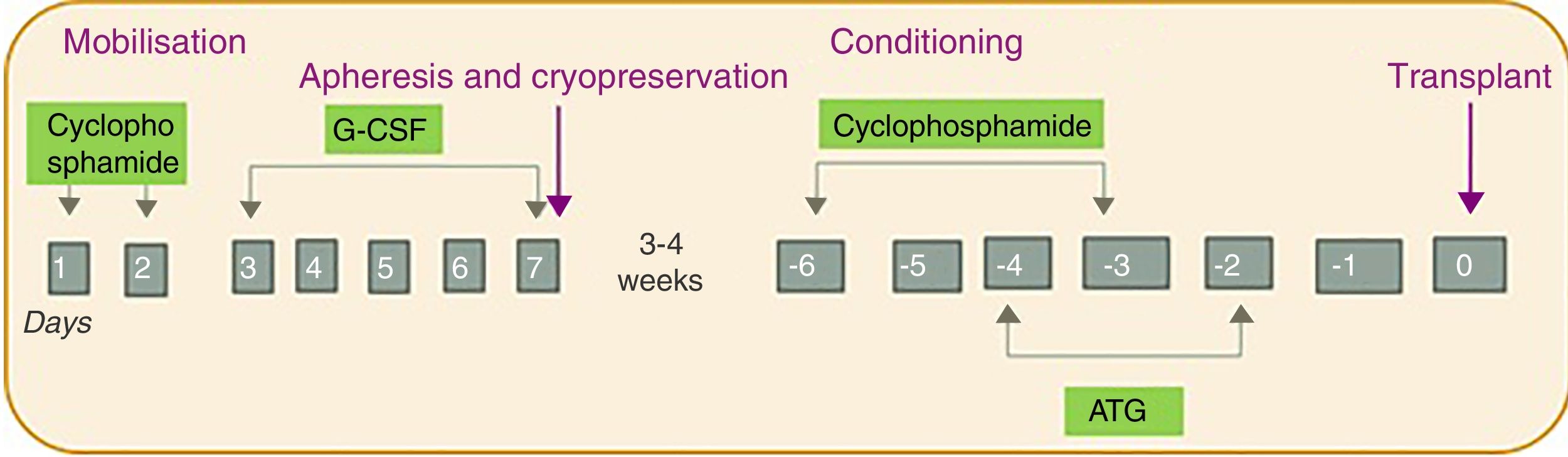
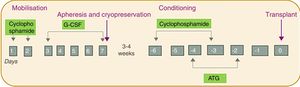
The patients all underwent AHSCT according to the same regimen involving four phases: (1) mobilisation; (2) harvesting and cryopreservation; (3) conditioning; and (4) transplant (Fig. 1). First was the mobilisation phase, with the administration of cyclophosphamide (dose of 2g/m2 for two days) and granulocyte colony stimulating factor (G-CSF; dose of 10μg/kg/day for five days). Next, the harvesting phase was carried out intravenously using a leukapheresis machine (model COBE Spectra), with a target of 2–4×106 CD34cells/kg. The cells were stored by cryopreservation until the transplant. For the cryopreservation phase, the cells obtained by leukapheresis are mixed with the cryoprotective solution dimethyl sulfoxide (DMSO), which is prepared at a final concentration of 10%. The preparation is carried out at <4°C in the laminar flow hood. The final volume of each bag should not exceed 150ml and the cell concentration should not exceed 2×108/ml. The final product is frozen in a freezer (model CM-2010), which follows a programmed and controlled freezing cycle, and stored at −80°C until the transplant. For the first two phases, the patients were kept in hospital for approximately two weeks. After 3–4 weeks, the conditioning phase was administered with cyclophosphamide (50mg/kg/day for four days) and equine or rabbit antithymocyte globulin (started two days after the cyclophosphamide for three days). Finally, the cells were transplanted. All this was carried out in conjunction with a supportive treatment protocol which included administration of broad-spectrum antibiotics before the first febrile peak, prophylactic antifungals and antivirals (fluconazole and acyclovir until the recovery of the neutrophil count), Pneumocystis jirovecii prophylaxis (trimethoprim-sulfamethoxazole for three months), as well as transfusion and nutritional support (use of total parenteral nutrition during the aplasia phase).
ResultsWe included patients with refractory CD who had undergone AHSCT at Hospital Universitario Ramón y Cajal in Madrid from June 2011 (date of completion of the first AHSCT in our centre) to 31 December 2017. We found a total of seven patients who had undergone AHSCT because of refractory CD. Since then, two further procedures have been carried out, taking us to a total of nine cases, but the last two are not included in this publication due to their short follow-up period.
The mean age at the time of the transplant was 26 (range: 16–43 years) and 5/7 (71%) were female. The average time from diagnosis of CD to the AHSCT was 9.9 years (range: 2.3–33.1 years).
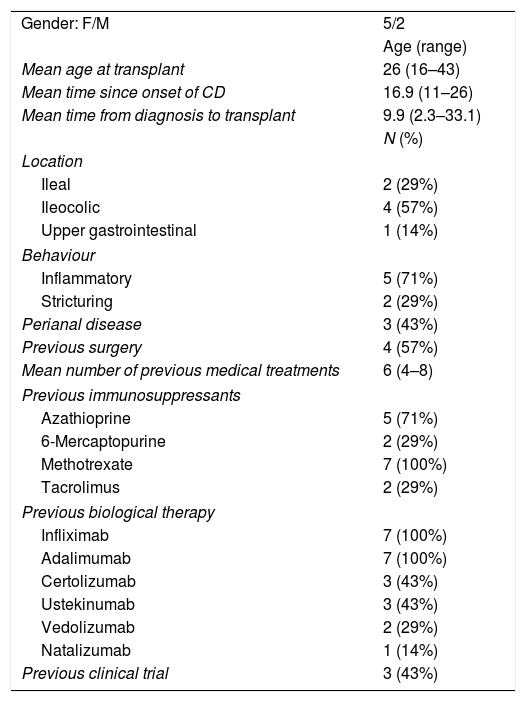
All the patients had previously received multiple treatments for CD, and had failed an average of six medical therapies (range: 4–8), and 57% had required surgery. A summary of previous treatments is shown in Table 1.
Characteristics of the patients and the CD, and previous treatments received.
| Gender: F/M | 5/2 |
| Age (range) | |
| Mean age at transplant | 26 (16–43) |
| Mean time since onset of CD | 16.9 (11–26) |
| Mean time from diagnosis to transplant | 9.9 (2.3–33.1) |
| N (%) | |
| Location | |
| Ileal | 2 (29%) |
| Ileocolic | 4 (57%) |
| Upper gastrointestinal | 1 (14%) |
| Behaviour | |
| Inflammatory | 5 (71%) |
| Stricturing | 2 (29%) |
| Perianal disease | 3 (43%) |
| Previous surgery | 4 (57%) |
| Mean number of previous medical treatments | 6 (4–8) |
| Previous immunosuppressants | |
| Azathioprine | 5 (71%) |
| 6-Mercaptopurine | 2 (29%) |
| Methotrexate | 7 (100%) |
| Tacrolimus | 2 (29%) |
| Previous biological therapy | |
| Infliximab | 7 (100%) |
| Adalimumab | 7 (100%) |
| Certolizumab | 3 (43%) |
| Ustekinumab | 3 (43%) |
| Vedolizumab | 2 (29%) |
| Natalizumab | 1 (14%) |
| Previous clinical trial | 3 (43%) |
CD: Crohn's disease; F: female; M: male.
All the patients received the AHSCT in accordance with the intervention protocol described in “Material and methods” section.
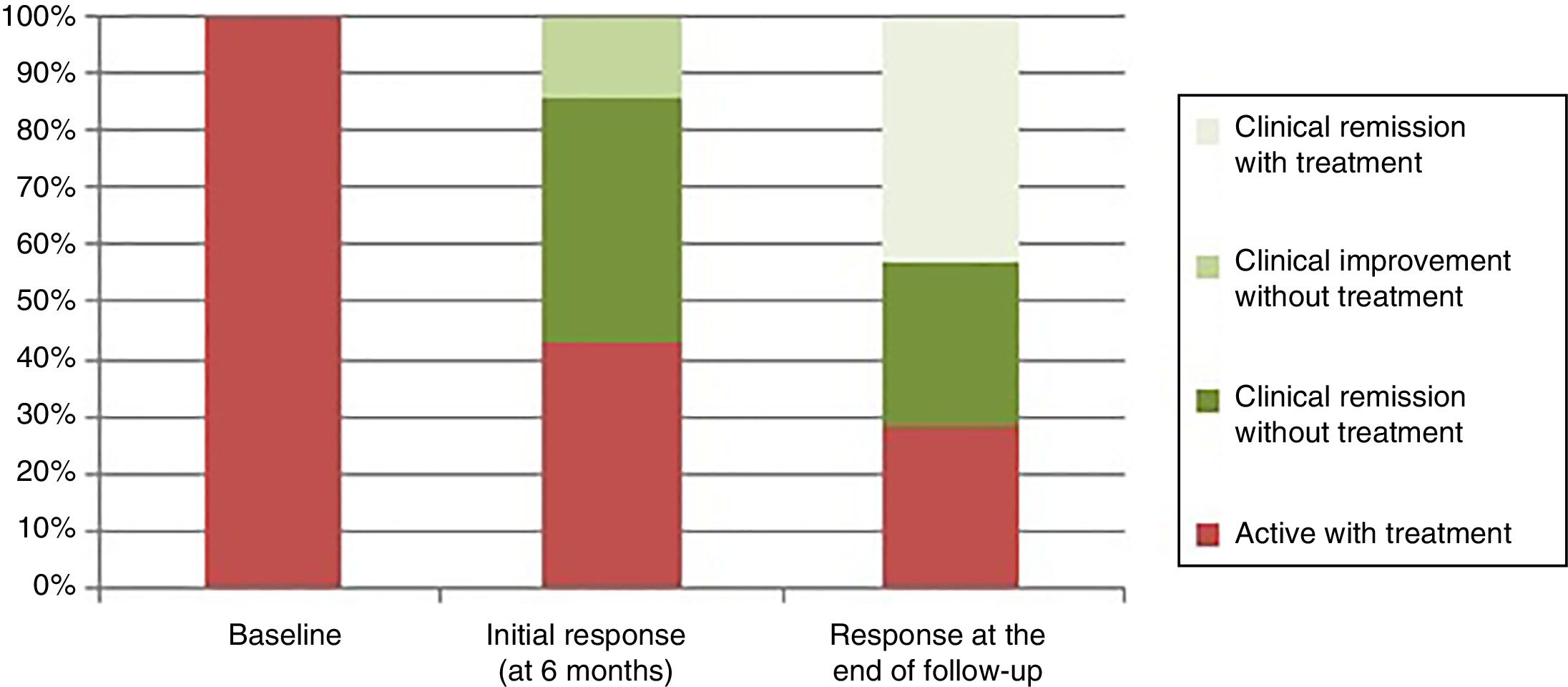
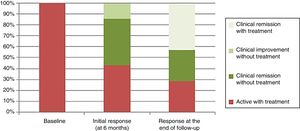
The clinical response was divided, according to the Physician Global Assessment by the treating physician, into clinical remission (absence of abdominal pain and normal bowel habit), clinical remission and endoscopic remission (mucosal healing of lesions in the endoscopy), improvement without remission (clinical improvement of the patient without achieving the criteria for clinical remission) and active disease (worsening of the patient's condition compared to the symptoms before the transplant). In the assessment of the initial response six months after the transplant, three patients (43%) had clinical and endoscopic remission, one patient (14%) clinical improvement without remission, and three patients (43%) had active disease after early relapse (one after three months and two after six months), and had to restart treatment, despite initial clinical improvement after the transplant (Fig. 2).
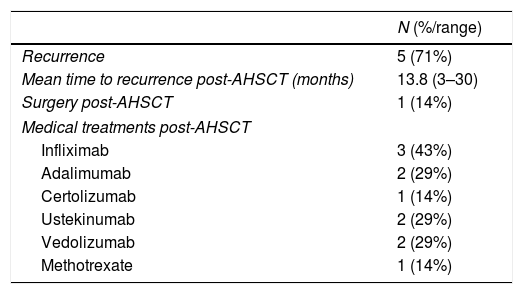
Overall, five out of seven (71%) of the patients had recurrence of symptoms and all five had to go back onto medical treatment (Table 2). Of the three patients who were in clinical and endoscopic remission in the initial assessment, two remained in remission and did not need to restart treatment, and one had disease recurrence 30 months after the AHSCT, which was treated with infliximab with good response. The patient with clinical improvement without remission six months after the transplant suffered recurrence 24 months post-transplant and required multiple treatments, but with no response. Of the patients with early recurrence: in one the recurrence occurred three months after the transplant, requiring treatment with methotrexate and infliximab, with good response, and two had recurrence after six months, one treated with adalimumab with good response and the other requiring multiple treatments, but with no response. Only one patient (14%) required surgery for recurrence of CD post-AHSCT.
Recurrence and treatments used post-autologous haematopoietic stem cell transplant (AHSCT).
| N (%/range) | |
|---|---|
| Recurrence | 5 (71%) |
| Mean time to recurrence post-AHSCT (months) | 13.8 (3–30) |
| Surgery post-AHSCT | 1 (14%) |
| Medical treatments post-AHSCT | |
| Infliximab | 3 (43%) |
| Adalimumab | 2 (29%) |
| Certolizumab | 1 (14%) |
| Ustekinumab | 2 (29%) |
| Vedolizumab | 2 (29%) |
| Methotrexate | 1 (14%) |
Mean follow-up was 48 months (range: 24–78 months). At the end of the follow-up the response was divided into active with treatment (2/7: 28.6%), clinical remission with treatment (3/7: 42.8%) and clinical remission without treatment (2/7: 28.6%) (Fig. 2).
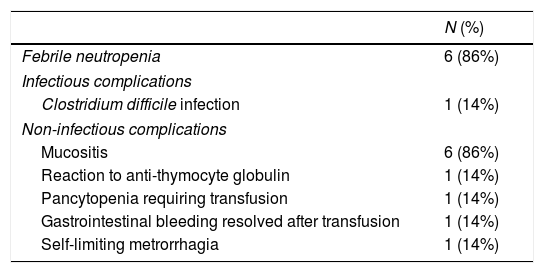
The most common post-transplant complications were febrile neutropenia and mucositis (6/7, 86% [Table 3]). No serious infectious complications were registered, with a single case of Clostridium difficile infection resolved with vancomycin treatment. No cases of cancer or development of other autoimmune diseases were described after AHSCT. There had been no deaths among the patients by the end of the follow-up period.
Post-autologous haematopoietic stem cell transplant complications.
| N (%) | |
|---|---|
| Febrile neutropenia | 6 (86%) |
| Infectious complications | |
| Clostridium difficile infection | 1 (14%) |
| Non-infectious complications | |
| Mucositis | 6 (86%) |
| Reaction to anti-thymocyte globulin | 1 (14%) |
| Pancytopenia requiring transfusion | 1 (14%) |
| Gastrointestinal bleeding resolved after transfusion | 1 (14%) |
| Self-limiting metrorrhagia | 1 (14%) |
In terms of outcomes, three patients had perianal disease prior to the transplant, but in all three this was under control at the time of the transplant. One patient had had a simple perianal fistula which was treated with antibiotics and adalimumab, with good progress two years after the AHSCT, and no evidence of fistula three years after the AHSCT. One patient had had a complex perianal fistula requiring surgery, with good outcome, four years before the AHSCT, and, one year after the AHSCT, she also showed no recurrence of the perianal disease. Lastly, one patient had a simple perianal fistula requiring a fistulotomy one year before the AHSCT, and three years after the AHSCT there was no evidence of perianal disease.
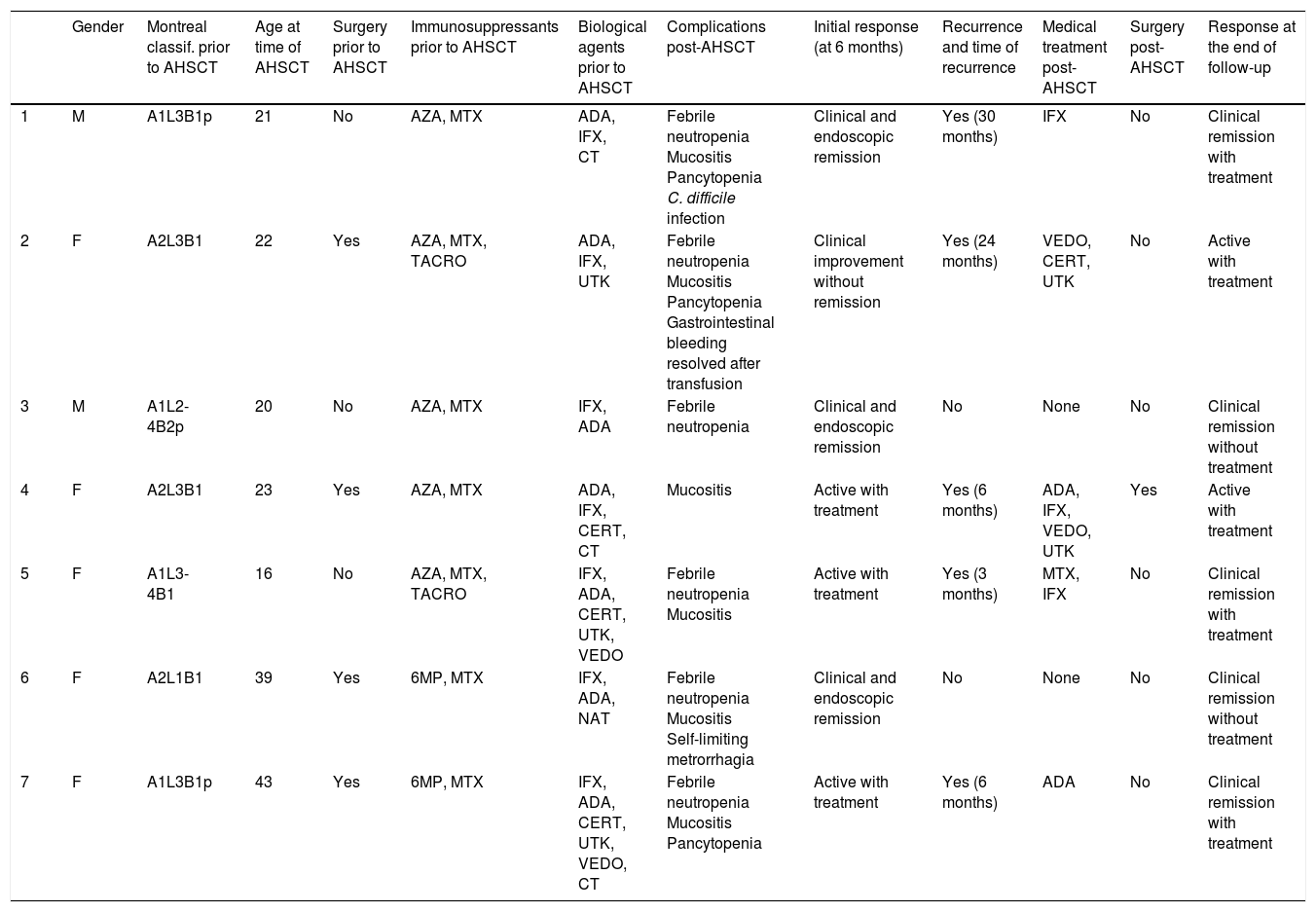
Table 4 shows a summary of the outcome for each patient.
Clinical progress of the patients included.
| Gender | Montreal classif. prior to AHSCT | Age at time of AHSCT | Surgery prior to AHSCT | Immunosuppressants prior to AHSCT | Biological agents prior to AHSCT | Complications post-AHSCT | Initial response (at 6 months) | Recurrence and time of recurrence | Medical treatment post-AHSCT | Surgery post-AHSCT | Response at the end of follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | A1L3B1p | 21 | No | AZA, MTX | ADA, IFX, CT | Febrile neutropenia Mucositis Pancytopenia C. difficile infection | Clinical and endoscopic remission | Yes (30 months) | IFX | No | Clinical remission with treatment |
| 2 | F | A2L3B1 | 22 | Yes | AZA, MTX, TACRO | ADA, IFX, UTK | Febrile neutropenia Mucositis Pancytopenia Gastrointestinal bleeding resolved after transfusion | Clinical improvement without remission | Yes (24 months) | VEDO, CERT, UTK | No | Active with treatment |
| 3 | M | A1L2-4B2p | 20 | No | AZA, MTX | IFX, ADA | Febrile neutropenia | Clinical and endoscopic remission | No | None | No | Clinical remission without treatment |
| 4 | F | A2L3B1 | 23 | Yes | AZA, MTX | ADA, IFX, CERT, CT | Mucositis | Active with treatment | Yes (6 months) | ADA, IFX, VEDO, UTK | Yes | Active with treatment |
| 5 | F | A1L3-4B1 | 16 | No | AZA, MTX, TACRO | IFX, ADA, CERT, UTK, VEDO | Febrile neutropenia Mucositis | Active with treatment | Yes (3 months) | MTX, IFX | No | Clinical remission with treatment |
| 6 | F | A2L1B1 | 39 | Yes | 6MP, MTX | IFX, ADA, NAT | Febrile neutropenia Mucositis Self-limiting metrorrhagia | Clinical and endoscopic remission | No | None | No | Clinical remission without treatment |
| 7 | F | A1L3B1p | 43 | Yes | 6MP, MTX | IFX, ADA, CERT, UTK, VEDO, CT | Febrile neutropenia Mucositis Pancytopenia | Active with treatment | Yes (6 months) | ADA | No | Clinical remission with treatment |
ADA: adalimumab; AZA: azathioprine; CERT: certolizumab; CT: clinical trial; IFX: infliximab; MTX: methotrexate; NAT: natalizumab; TACRO: tacrolimus; UTK: ustekinumab; VEDO: vedolizumab; 6 MP: 6-mercaptopurine.
Although this is a small series of patients, our results suggest that AHSCT may be a treatment option for patients with refractory CD. When assessing the initial response, almost half of the patients (3/7, 43%) were in clinical and endoscopic remission or showed clinical improvement (1/7, 14%) with no need for treatment. Three patients (3/7, 43%) remained active with a need to restart treatment six months after the transplant. The majority of patients (5/7, 71%) suffered recurrence and had to restart treatment. However, at the end of the follow-up (at an average of 48 months) the majority of patients (5/7, 71%) were in clinical remission with or without treatment. In the only randomised trial7 published to date, the primary endpoint was not achieved, but it had probably been made too strict (CDAI<150 with endoscopic mucosal healing at one year and no active treatment in the last three months); even stricter than the objectives used when assessing conventional therapy. However, there did seem to be a benefit in the secondary endpoints of clinical improvement (CDAI), endoscopic improvement (Simple Endoscopic Score for Crohn's Disease) and quality of life. Two non-randomised trials were subsequently published which supported the use of AHSCT in refractory CD.13,14 The only Spanish series, which is the largest series from a single centre published to date,13 prospectively included 29 patients (not included in the ASTIC study). The percentage of patients in clinical and endoscopic remission without treatment post-transplant was found to be decreasing with the passage of time: 61% at one year, 47% at three years, and 15% after five years. However, the patients who suffered recurrence were treated again, and the remission rate with the treatment was 80%. Five years after the AHSCT, 100% of the patients were in remission, with or without treatment.13 Similar data had previously been obtained in the Chicago series, with the proportion of patients not requiring treatment being 91% at one year, 57% at three years and 19% five years after the AHSCT.10 However, five years after the AHSCT, 80% of the patients were in remission, with or without treatment.10 A recent study presented at the 2018 European Crohn's and Colitis Organisation congress14 reports the results of the largest existing cohort (82 patients from seven European countries not included in the ASTIC study); 67% of the patients showed complete remission or clinical improvement after the AHSCT (at a mean follow-up of three years). Most of the patients required reintroduction of treatment to achieve clinical improvement and, in 57%, a response was obtained to treatments to which they had previously lost response.14 From the above evidence, it would seem that the utility of AHSCT lies in the fact that it can produce remission in some patients, but also that it can help patients achieve clinical remission in response to drugs which had previously been ineffective or to which they had previously lost their response. Any evaluation of its efficacy has to include the fact that patients who reach this stage have already gone through most of the available therapies, and are not good candidates for surgery, either because of the extent of their disease or the fact that previous surgical interventions complicate any further surgery.
The main limitation of the use of AHSCT in CD is its toxicity. AHSCT has an estimated mortality rate of 1.2%.6 In our study, the most common post-transplant complications were mild, such as febrile neutropenia and mucositis (6/7, 86% [Table 2]). No fatal complications or serious infectious complications were registered, with a single case of Clostridium difficile infection who recovered well with treatment. In the ASTIC study, one patient died as a result of sinusoidal obstruction syndrome 20 days after the conditioning phase.7 In the cohort presented at the European Crohn's and Colitis Organisation, one patient died of cytomegalovirus infection on day 56 post-AHSCT and another died eight years after the AHSCT of a cause unrelated to the transplant.14 Infections were the most common post-AHSCT complication (27%), with those of viral origin being the most common (13%).14 This finding was also documented in the Barcelona series: 38% of the patients had infection with viruses from the herpes family and one patient died as a result of a disseminated cytomegalovirus infection two months after the transplant.13 It would seem that optimising the support treatment during the mobilisation and conditioning phases can improve the infectious complication rate.13,15 There are limited data on the possible long-term complications of AHSCT in refractory CD. In the Barcelona study, 13% of the patients developed another autoimmune disease, and 6% some form of cancer, during the post-AHSCT follow-up period.13 With all these aspects in mind, it seems most appropriate that AHSCT should be carried out in specialised centres, with careful selection of patients.16
We would conclude that AHSCT appears to be a useful treatment option in patients with refractory CD, as it can achieve clinical remission and recover the response to certain treatments in patients who had previously lost response. Nonetheless, controlled randomised trials are still needed in this field. One of the biggest limitations of AHSCT is its toxicity. Patients should therefore be well selected and the intervention should take place in specialised centres. Studies with a longer follow-up are necessary to clarify long-term side effects.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Hernanz N, Sierra M, Volpato N, Núñez-Gómez L, Mesonero F, Herrera-Puente P, et al. Trasplante de precursores hematopoyéticos en enfermedad de Crohn refractaria: experiencia en nuestro centro. Gastroenterol Hepatol. 2019;42:16–22.