Chronic pancreatitis is a chronic fibroinflammatory disease of the pancreas with prevalence around 50 cases per 100,000 inhabitants. It appears to originate from diverse and yet mixed etiological factors. It shows highly variable presenting features, complication types and disease progression rates. Treatment options are as wide as the multiple personalized scenarios the disease might exhibit at a given time point. Some medical societies have developed guidelines for diagnosis and treatment based on scientific evidence. Although these efforts are to be acknowledged, the gathered level of evidence for any topic is usually low and, therefore, recommendations tend to be vague or weak.
In the present series of position papers on chronic pancreatitis from the Societat Catalana de Digestologia and the Societat Catalana de Pàncrees we aimed at providing defined position statements for the clinician based on updated review of published literature and on interdisciplinary expert agreement. The final goal is to propose the use of common terminology and rational diagnostic/therapeutic circuits based on current knowledge. To this end 51 sections related to chronic pancreatitis were reviewed by 21 specialists from 6 different fields to generate 88 statements altogether. Statements were designed to harmonize concepts or delineate recommendations. Part 1 of this paper series discusses topics on aetiology and diagnosis of chronic pancreatitis. Main clinical features are abdominal pain, exocrine and endocrine insufficiency and symptoms derived from complications. Some patients remain symptom-free. Diagnosis (definitive, probable or uncertain) should be based on objective data obtained from imaging, histology, or functional tests.
La pancreatitis crónica es una enfermedad fibroinflamatoria del páncreas originada por acción combinada de factores etiológicos. Muestra formas de presentación, tipos de complicaciones y grados evolutivos variables. Las opciones terapéuticas son tan diversas como los múltiples escenarios clínicos. Algunas sociedades médicas han desarrollado guías sobre diagnóstico y tratamiento basadas en evidencia científica. Pero la elevada variabilidad que conforman la conjunción de elementos etiológicos, presentaciones clínicas, complicaciones y progresión de la enfermedad hace que los niveles de evidencia obtenidos sean generalmente bajos y, por tanto, las recomendaciones tienden a ser vagas o débiles, salvo excepciones.
En los presentes documentos de posicionamiento de la Societat Catalana de Digestologia y la Societat Catalana de Pàncrees hemos buscado redactar declaraciones bien definidas orientadas al clínico, basadas en revisiones actualizadas de literatura y acuerdos de expertos. El objetivo es proponer el uso de terminología común y circuitos diagnóstico/terapéuticos racionales basados en el conocimiento actual.
Para este fin se revisaron 51 secciones relacionadas con pancreatitis crónica por 21 expertos de 6 especialidades diferentes para generar finalmente 88 declaraciones que buscan armonizar conceptos y formular recomendaciones precisas.
La parte 1 de esta serie de documentos discute tópicos sobre etiología, diagnóstico y diagnóstico diferencial. Factores etiológicos de mayor relevancia son tóxicos (alcohol y tabaco), genéticos y obstructivos. Dolor abdominal, insuficiencia exocrina y endocrina y síntomas derivados de complicaciones son las presentaciones más frecuentes. Algunos pacientes permanecen asintomáticos. El diagnóstico (seguro, probable o incierto) debe sustentarse en datos objetivos obtenidos en pruebas de imagen, histología y pruebas de función pancreática.
This is the first of a series of 3 documents aiming at highlighting the main concepts and actions recommended by the Societat Catalana de Digestologia [Catalan Gastroenterology Society] (SCD) and the Societat Catalana de Pàncrees [Catalan Pancreas Society] (SCPanc) on clinical topics of chronic pancreatitis (CrP). The goal is to propose a common terminology and rational diagnostic and therapeutic circuits based on current knowledge. Documents 2 and 3 will be focused on treatment and follow-up (Part 2) and on complications and special forms of the disease (Part 3).
To this end, articles and their relevant references published in the last 6 years regarding 51 sections related to chronic pancreatitis were reviewed by 21 specialists working in the disciplines of gastroenterology (8), endoscopy (4), surgery (4), pathology (2), radiology (2) and endocrinology (1) at 11 large public hospitals in Catalonia. The specialists were asked to prioritize details of practical clinical application in their conclusions and recommendations. From the updated review of the sections, a preliminary document was drawn up that was discussed by the entire group. Whenever discrepancies among group members were detected, the text was edited in order to achieve a consensus greater than 90%. The document was later presented to the two sponsoring societies for their approval.
Phrases preceded by the abbreviation SCD-SCPanc indicate the position of the two organizations in the different statements, either to emphasize a concept or to express a recommendation. Many sections deserve to be covered in independent review articles by their own, but this challenge lay beyond the scope of the present reviews.
DefinitionSCD-SCPanc 1. Chronic pancreatitis is a chronic inflammatory process of the pancreas that leads to fibrosis and loss of the exocrine and endocrine parenchyma (atrophy).
The precise definition of CrP continues to be the subject of debate which stretches fundamental concepts. The core of the definition is usually the one expressed above, but some experts tend to incorporate variations, such as including pathogenic (mechanistic) theories in the definition, describing CrP as a syndrome, or demanding clinical or prospective events. The main reason for these more elaborated definitions is to be able to differentiate truly CrP from morphological irrelevant alterations that may appear in particular situations (advanced age, smokers, diabetes mellitus, ….). This discussion is important because it establishes boundaries to be respected in epidemiological, clinical and pharmacological studies. The SCD and SCPanc do not oppose to these more complex definitions, but consider that the definition articulated here is the simplest one, it concentrates the essence of the disease, allows the inclusion of asymptomatic forms, and admits the possibility that the disease may slow its progression under favourable conditions (for example, after elimination of causative factors).
With regard to other definitions requiring particular clarification, we consider the following for this series of documents:
Recurrent pancreatitis (RAP): a minimum of two well-documented acute pancreatitis episodes separated by at least 3 months from each other with total recovery (functional and morphological) in the interval, and without evidence of CrP.1,2
Hereditary pancreatitis: pancreatitis that affects two or more individuals in two or more generations of a family without a known etiological factor. Some authors include pancreatitis associated with the presence of pathogenic genetic mutations, although manifestations in other members of the family were vague or non-existent.3 When the strict conditions of hereditary pancreatitis are not met there is a trend to designate the association as familial pancreatitis.
Exocrine pancreatic insufficiency (EPI): Functional impairment of pancreatic enzyme and bicarbonate secretion to a notable degree, regardless of the cause.
EpidemiologySCD-SCPanc 2. The incidence of CrP ranges between 5 and 10 cases per 100,000 inhabitants/year and the prevalence around 35–50 cases per 100,000 inhabitants.
The published figures are rough calculations and vary great deal. Original data usually come from studies with limited reliability because they depend on the population studied, the method of patient selection, and the diagnostic accuracy of the disease.4–6
Some groups report an incidence of 120 cases/100,000 inhabitants. If it only was 10/100,000, the 20-year prevalence should be 200/100,000. Lower figures suggest that patients have high mortality or that our diagnostic accuracy is poor. Incidence is higher in men.4,7 In the United States, the mean age at diagnosis is 56 years.8 37% of patients are diagnosed before the age of 35 and 11% after the age of 65.9
Hospital admissions for acute pancreatitis and complications of the disease are common.6 However, only 50% of patients refer well-documented previous episodes of pancreatitis.6
Some patients remain asymptomatic, but their precise prevalence is unknown. They may comprise 5–10% of all patients with CrP.10
EtiologySCD-SCPanc 3. CrP is thought to be caused by one or multiple factors acting on subjects with individual predisposition. It is unusual that a single factor fully accounts for CrP generation.
SCD-SCPanc 4. Risk of developing CrP increases with the association of factors, the specific weight of each factor in each individual, and time of exposure. Eliminating risk factors can slow down the progression of the disease.
SCD-SCPanc 5. We consider pancreas divisum can be viewed as an etiological factor if the following conditions are present:
A Morphological disease confined to the dorsal pancreas (by EUS or MRI); or
B Outlet obstruction to pancreatic flow through the papilla minor, as demonstrated by the presence of santorinicele or by dilatation >3 mm of the duct of Santorini following secretin infusion.
The currently most accepted view of CrP pathogenesis is that it develops as a result of the action of one or, most frequently, multiple aggressive factors acting for some prolonged time. Table 1 (modified from version 1 TIGAR-O11) lists the possible etiological factors, and it can be used as a guide to review them in any patient recently diagnosed with CrP.
Etiological/risk factors for chronic pancreatitis based on the TIGAR-O V1 classification11 with minor modifications.
| Toxic-metabolic |
| Alcohol |
| Smoking |
| Hypercalcemia (ionic calcium ≥12 mg/dL) |
| Hypertriglyceridemia |
| Drugs (possible) |
| Toxins |
| Stage 5 chronic kidney disease |
| Factors associated with oxidative stress (radiotherapy/chemotherapy, ischemia) |
| Metabolic causes (diabetes mellitus, obesity) |
| Idiopathic |
| Genetic |
| PRSS1 |
| CFTR |
| SPINK1 |
| CTRC |
| CPA1 |
| CEL or CEL-HYB |
| CLDN2 locus |
| TRPV6 |
| Autoimmune pancreatitis types 1 and 2 |
| Recurrent pancreatitis and severe acute pancreatitis |
| Any etiology |
| Obstructive |
| Pancreas divisum and other anatomical abnormalities |
| Ampullary stenosis |
| Intraductal calculi in pancreatic main duct |
| Wirsung duct stenosis |
| Intraductal papillary mucinous neoplasm |
| Obstructive pancreatic mass |
Consumption of tobacco and alcohol (Table 1) are independent risk factors, but few individuals develop the disease (<5% of heavy alcohol users).12,13 Risk is only significant in patients who maintain a high and sustained consumption of alcohol or are chronic smokers. Even in these individuals it is recommended to carry out a global etiological assessment that should take into account other etiological factors. For instance, combining alcohol and CLDN2 mutations14–16 or tobacco and CFTR or CTRC mutations increase the incidence of CrP.17 All these possible combinations acting on a given individual explain, to a large extent, the enormous variable susceptibility of alcohol and tobacco in induction and progression of CrP.
Alcohol increases the risk of developing CrP in a dose and time-dependent manner, as well as the progression rate.18 Persistence of consumption after an episode of acute pancreatitis facilitates recurrences and progression to CrP.19,20
The tobacco-associated risk is also dose-dependent. It is maintained in ex-smokers to a lesser extent. It is independent of alcohol consumption.13,21,22
A high percentage of patients with CrP carry pathogenic mutations in specific genes.13,23 The most frequent mutated genes in our environment that relate to CrP are CFTR (cystic fibrosis transmembrane conductance regulator) and SPINK1 (serine protease inhibitor kazal-type 1), and less frequently CTRC (Chymotrypsin-C), CPA1 (carboxypeptidase A1) and PRSS1 (cationic trypsinogen). Some variants in CLDN2 (claudin 2) are also associated with CrP.
Genetic mutations are highly prevalent in paediatric patients (55–73%),24,25 in idiopathic CrP (48–57%) and in early onset forms (<35 years of age).26–28 They are also increased in CrP associated with alcohol and tobacco,14,15,26 which reinforces the concept that CrP is multifactorial disease. Individuals with pancreas divisum have a high incidence of mutations in CFTR and SPINK1.29
Hereditary CrP usually onset in childhood or early adulthood. It shows rapid evolution towards chronicity and carries a high risk of pancreatic cancer.30,31
Mutations in PRSS1 induce the best characterized form of hereditary pancreatitis, although it has a low incidence in our environment. They show autosomal dominant inheritance with incomplete penetrance (80%) that usually affects several members of a family.32,33 They also cause sporadic CrP.34
30% of patients with CrP carry CFTR mutations, a much higher prevalence than in healthy population. These mutations induce great phenotypic variability, from asymptomatic carriers to cystic fibrosis (CF).35 This variability is determined by the residual function of the CFTR protein, which depends on the type of mutation (see cftr2.org) and the concurrence of other etiological factors, specifically alcohol and tobacco.
The association of 2 severe mutations or 1 severe and another mild in different alleles determines the development of CF, which involves 2 or more typical organs (particularly the lungs and pancreas) and shows sweat chloride concentration >60 mM.36 Pancreatic involvement is almost universal in the form of atrophy, recurrent or chronic pancreatitis. In some patients with known CrP we will have to consider the differential diagnosis with CF (in its non-classical form), which in some countries would entail healthcare benefits for the patient. Diagnosis is based on detection of pathological sweat test, pathogenic mutations in the two alleles and/or involvement of more than one target organ.
On the other hand, mutations in CFTR are common in idiopathic CrP.27,28,37,38 Some non-causing CF variants (R74Q, R75Q, R117H, R170H, L967S, L997F, D1152H, S1235R, and D1270N) predominantly affecting bicarbonate transport increase the risk of CrP (OR 1.5).38
The most frequent mutation in SPINK1, N34S, is found in 9.7% of patients (up to 30% of idiopathic forms) compared to 0.07% of healthy individuals (OR 11). Risk is higher in tropical CrP (OR 19.15).39 The c.194 + 2T > C mutation affects 3% of idiopathic CrP in the West, but up to 50% in some Asian populations (OR 30.4 in heterozygous and 162 in homozygous).26 Other mutations in SPINK1 (intronics, deletions, etc.) are rarer, but usually severe.
Mutations in CTRC (R254W, K247_R254del, A73T, V235I) have been described in 4% of patients with non-alcoholic CrP (0.7% in the control population), which translates into a x5 risk.40 The G60G variant (c.180 C > T/A) is identified in 17–30% of CrP, conferring a risk of x2.5 in heterozygosity and x10 in homozygosis.41,42 It has been described more frequently in smokers with CrP (22.6%) or associated with mutations in CFTR or SPINK1 (22.9%).17
Mutations in CPA1 are present in 3% of CrP (0.3% in controls) with an OR of 25, higher in childhood (OR 80).43
Pathogenic mutations in the TRPV6 gene (channel involved in calcium absorption) have recently been reported in 2–4% of patients with idiopathic chronic pancreatitis in Japan and Europe (OR 48).44
The association of mutations in the same gene or in different genes (e.g. CFTR and SPINK1) increases the risk of CrP.23,40 On the other hand, tobacco, alcohol and autoimmunity can reduce CFTR expression and function in the absence of mutations.45–47 Tobacco increases the risk of CrP associated with most mutations.
Ductal obstruction due to tumours, cysts or injury secondary to necrotizing pancreatitis or trauma favours development of CrP proximal to the obstruction.
There is no consensus on accepting sphincter of Oddi dysfunction or pancreas divisum as isolated causes of CrP, although this debate has not been settled.
Pancreas divisum is a variant of the ductal system that can be present in 10% of healthy people, in which the ventral duct does not fuse with the dorsal duct (complete divisum) or there is a patent Santorini duct (incomplete divisum). The most sensitive techniques for its detection are endoscopic ultrasonography (EUS) and magnetic resonance imaging (MRI) (ideally with secretin48). Some studies have observed a higher incidence of pancreas divisum in patients with RAP or CrP than in the general population (up to 43% in a Japanese study), but in many other series no differences were detected.49,50
Patients with pancreas divisum and RAP or CrP have a higher prevalence of SPINK1 or CFTR mutations, or just abnormal CFTR function.28,49,51,52 Furthermore, both tobacco and alcohol suppress the expression of CFTR. Most of the studies investigating the relationship of pancreas divisum with CrP do not report on the mutational status of patients or their toxic habits.
Autoimmune pancreatitis (AIP) is a special form of CrP that will be better discussed in Part 3 of this series. It is characterized by having a good response to corticosteroids.53 Morphological features of long term AIP are indistinguishable from other forms of CrPs.
Hypocalcaemia, hypertriglyceridaemia, and some drugs are controversial and rare causes of CrP. The mechanism appears to involve repeated outbreaks of severe pancreatitis.
Other factors such as diabetes, obesity, end-stage renal failure, metabolic syndrome, radiotherapy/chemotherapy, and ischemia have been associated with CrP in epidemiological studies or in isolated cases, but their etiopathogenesis remain unknown. This association is best documented for diabetes mellitus.54
In 10%–20% of patients no causative factor can be identified (idiopathic pancreatitis).
Clinical manifestationsSCD-SCPanc 6. The main clinical manifestations of CrP are abdominal pain and exocrine and/or endocrine pancreatic insufficiency, but some patients may remain asymptomatic.
SCD-SCPanc 7. Clinical presentation in the form of acute or recurrent pancreatitis or in the form of complications is not uncommon.
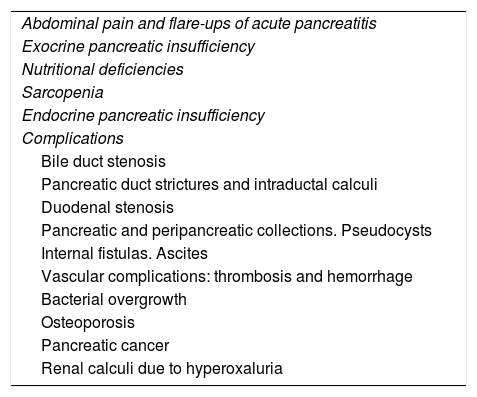
Clinical manifestations associated with CrP are diversed.55 From an academic view for this series of documents on CrP we will consider abdominal pain, exocrine insufficiency, nutritional deficiencies and endocrine insufficiency as manifestations of the disease, while we will refer to other associated manifestations such as complications (Table 2), though this differentiation is artificial.
Clinical manifestations of chronic pancreatitis.
| Abdominal pain and flare-ups of acute pancreatitis |
| Exocrine pancreatic insufficiency |
| Nutritional deficiencies |
| Sarcopenia |
| Endocrine pancreatic insufficiency |
| Complications |
| Bile duct stenosis |
| Pancreatic duct strictures and intraductal calculi |
| Duodenal stenosis |
| Pancreatic and peripancreatic collections. Pseudocysts |
| Internal fistulas. Ascites |
| Vascular complications: thrombosis and hemorrhage |
| Bacterial overgrowth |
| Osteoporosis |
| Pancreatic cancer |
| Renal calculi due to hyperoxaluria |
Continuous or intermittent pain affects >75% of patients and has a significant impact on their quality of life. Pain is typically intense, epigastric, radiates to the sides and worsens with meals. Sometimes it radiates to the back. Vomiting may arise, but if this is persistent, we should suspect gastric outlet obstruction. Intermittent pain is even more common than continuous pain, with long periods without pain. Pain is often associated with bouts of pancreatitis, particularly if associated with genetic factors, alcohol/tobacco, ductal obstruction, or groove pancreatitis. CrP is one of the most relevant causes of recurrent acute pancreatitis.
Other causes of CrP-related pain are duodenal obstruction, complicated pseudocysts, peptic ulcers, hemosuccus pancreaticus, and pancreatic cancer. Bile duct obstruction only causes pain if there is associated lithiasis or acute cholangitis.
In some patients abdominal pain is not a relevant symptom or may be totally absent.
Exocrine insufficiency (EPI)The degree of EPI is highly variable and correlates with the stage of the disease. It is more frequent after pancreatic surgery (pancreatectomies, necrosectomies).56 The spectrum ranges from partial vitamin or micronutrient deficiencies to frank steatorrhea with weight loss.57,58
In mild cases, patients may remain asymptomatic or report abdominal discomfort, bloating, or meteorism. With higher degrees of EPI loose stools or diarrhoea may become apparent, as well as malnutrition (hypocholesterolemia, hypoalbuminemia) and weight loss that occurs with a preserved appetite. Steatorrhea usually presents in advanced stages, sometimes with oily stools. In advanced EPI, serum levels of pancreatic enzymes are usually low, and sometimes they become undetectable.
Nutritional deficienciesExocrine and endocrine insufficiencies contribute to malnutrition, but also reduced food intake (due to alcoholism or to avoid abdominal pain) and accelerated catabolism (due to chronic inflammation). Vitamin D deficiency can affect 40–66% of patients. It is associated with osteopenia (42%) and osteoporosis (39%), to which vitamin K deficiency also contributes. 35% of patients have vitamin A deficiency, and 18% vitamin E deficiency. Patients who smoke have a higher incidence of vitamin D and E deficiency. Vitamin deficiencies can lead to osteoporosis, anaemia, dermatitis, coagulopathy, visual disturbances, and neuropathy.59–61
Some patients have acceptable exocrine function tests and nutritional parameters, but may carry defects in elements such as ferritin, prealbumin, calcium, magnesium, zinc, selenium, thiamine, vitamins B6 and B12, folic acid, manganese, copper, sulphur, riboflavin, and choline.
SarcopeniaThis is the loss of muscle mass and function. Muscle strength is assessed using a dynamometer and muscle mass is assessed through imaging tests (CT or MRI) or biometric impedanciometry.
It affects up to 20% of patients, independent of body mass index (BMI). It is usually a consequence of EPI62,63 that may have been indolent and under-treated. It is predictor of postsurgical complications.
Endocrine insufficiencyIt can be the first manifestation of the disease and may be misinterpreted as some more common forms of diabetes, but it has particular characteristics: patients do not usually present obesity, nor do they meet diagnostic criteria for type 1 diabetes mellitus (negative autoimmunity). Although insulinopenia is more characteristic, insulin resistance may be present.
This form of diabetes has been labelled pancreopriva, pancreatogenic, diabetes of the exocrine pancreas, or type 3c diabetes mellitus.57,64 It represents only 1–8% of all diabetes mellitus, but almost 80% of patients with CrP will develop it sooner or later. It is more common in tropical pancreatitis.
The risk of diabetes increases with the duration of the disease, the evolutionary stage and after pancreatectomies. Islet destruction, inflammation, autoimmunity, genetic alterations and enolism are involved in its etiopathogenesis.
Associated deficiencies of glucagon and pancreatic polypeptide add difficulty in the control of glycaemia, making hypoglycaemia a frequent event. If some degree of insulin resistance is also present, it becomes clear why therapeutic control of these patients is particularly difficult.
ComplicationsIt is worth to remember that patients with CrP tend to develop complications that can dominate the clinical picture (Table 2) and even be the form of presentation of the disease. The discussion of these complications will be the objective of Part 3 in this series.
DiagnosisSCD-SCPanc 8. The patient's symptoms may suggest the presence of chronic pancreatitis, but diagnosis must be established with objective data, indicating the degree of diagnostic evidence achieved and the means used. Equally important is to exclude CrP in a reasoned way, or to state that there is not enough evidence available to establish a formal diagnosis.
SCD-SCPanc 9. The objective data necessary for diagnosis are obtained by radiological or endoscopic imaging tests, histological examination of pancreatic tissue, and exocrine and endocrine function tests.
The most frequent causes of abdominal pain or chronic diarrhoea are not of pancreatic origin. Furthermore, establishing the diagnosis of CrP carries a significant healthcare burden: it requires investigating the aetiology and eliminating associated factors, attending current or future complications, considering patient's quality of life, and anticipating development of cancer in selected groups.
Endoscopic retrograde cholangiopancreatography is no longer used for the diagnosis of CrP as it has been replaced by imaging techniques with less iatrogenic potential. However, its use led to the development of the Cambridge classification, still in use, which categorizes the findings in imaging tests and correlates them with the stage of the disease.
Radiological imaging techniques: computerized tomography (CT), magnetic resonance (MRI), ultrasoundSCD-SCPanc 10. For strategic reasons, we use CT as the initial study technique, but MRI is perfectly reliable. The study protocol should include administration of intravenous contrast and additional image acquisition in the portal phase, although initially a simple study and a late arterial phase are usually combined. The MRI protocol should include, in addition to the standard examination with morphological sequences to evaluate the parenchyma, MRCP sequences that allow for detailed evaluation of the ductal anatomy.
Imaging techniques are of relative value in the early stages of the disease. CT and MRI (conventional or MRCP-cholangiopancreatography) are the most widely used techniques. They have a high and comparable diagnostic accuracy in well-established CrP. Findings should be reported in a standardized way.
Abdominal ultrasound is of great value in the paediatric population.65–68
The intravenous administration of secretin (1 mL/10 kg of body weight; MRCP-s) allows for better visualization of the ductal tree and for indirect estimation of the exocrine function by evaluating the degree of excretion of pancreatic juice to the duodenum.68 It has been suggested that the absence of dilatation of the pancreatic duct in response to secretin would be an early sign of CrP that would reflect a loss of elasticity due to increased rigidity of the walls.
Signs of established CrP on imaging- 1
Parenchymal signs
- -
Calcifications. Best detected by CT, but some are radiotransparent. The prevalence is variable. Calcifications are not specific to CrP and can appear in certain tumours (intraductal papillary mucinous neoplasia, neuroendocrine tumours, serous cystadenoma) and vascular calcifications in elderly patients can be mistaken for parenchymal calcifications. They are more specific to CrP if diffusely present in the parenchyma and in the ducts.
- -
Pancreatic atrophy. This is the most frequent finding (54%), but it is not very specific and can be confused with senile changes.
- -
Signal intensity. Decreased parenchymal signal in T1-weighted sequences with fat saturation is an early sign that may precede ductal changes.67
- -
Magnetic resonance elastography, T1 or multiparametric mapping, and extracellular volume quantification are quantitative methods that may be useful in early diagnosis and staging.69
- -
Contrast enhancement. The enhancement pattern and its quantification correlate with the degree of fibrosis. It has a high sensitivity in early stages. Contrast uptake in CrP is heterogeneous, delayed and decreased in the early arterial phase and more persistent in the late phase.70
- -
Irregular gland contour
- -
- 2
Ductal signs
The best non-invasive technique is MRI.71 Several types of findings are described: dilation/stenosis of the main pancreatic duct (which may be beaded) and dilation of the lateral ducts. Intraductal stones, pseudocysts or anatomical variants can also be observed.67 We reserve secretin-enhanced MRI for cases in which there is high suspicion of CrP but conventional imaging tests (including EUS) are not conclusive.
A modification of the Cambridge classification has been proposed to diagnose and stage CrP using CT and MRI.65,72 The most specific initial finding is the presence of >3 dilated secondary ducts, even better if the main duct also appears dilated and irregular.
Endoscopic ultrasonography (EUS)SCD-SCPanc 11. EUS detects very early changes, but the findings must be interpreted with caution if other imaging or functional tests are normal.
SCD-SCPanc 12. If <2 EUS criteria are found the diagnosis of CrP is unlikely, while if >5 are detected diagnosis is highly probable. If 3 or 4 criteria are found the diagnosis is indeterminate.
EUS is very sensitive in detecting abnormalities in the pancreas. However, the interobserver agreement is not very good, and many anomalies lack specificity as they are also detected in asymptomatic patients with anatomical variants, the elderly, obese, diabetics, smokers, alcoholics, pancreatic steatosis, and during recovery from acute pancreatitis. For this reason, we must be cautious when establishing the diagnosis of CrP solely based on EUS.
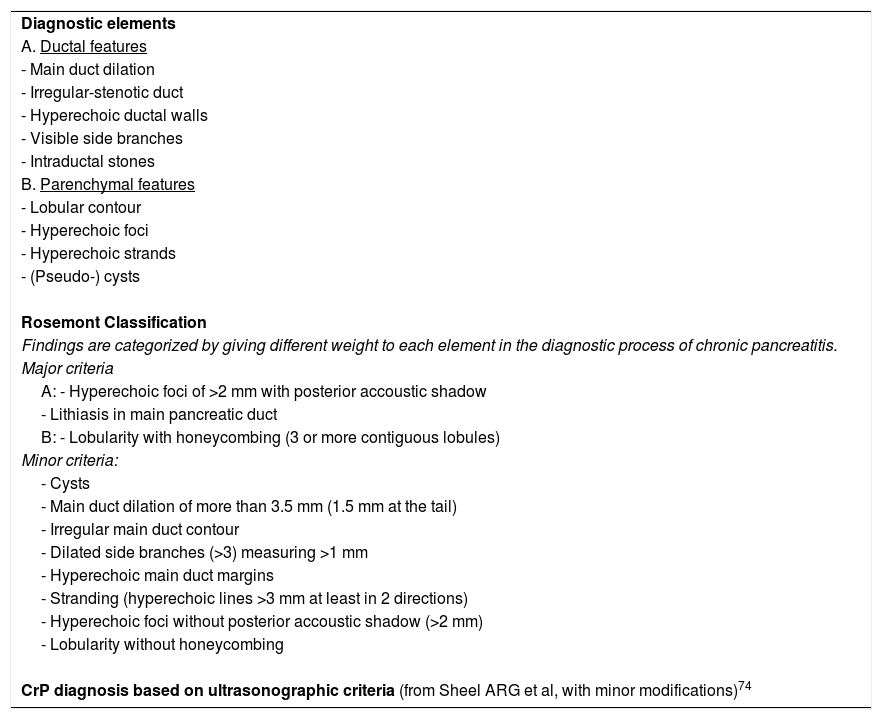
EUS allows for good visualization of the parenchyma and pancreatic ducts. Up to 9 potentially abnormal ductal and parenchymal criteria are described (Table 3).73
Ultrasonography in the diagnosis of chronic pancreatitis.
| Diagnostic elements |
| A. Ductal features |
| - Main duct dilation |
| - Irregular-stenotic duct |
| - Hyperechoic ductal walls |
| - Visible side branches |
| - Intraductal stones |
| B. Parenchymal features |
| - Lobular contour |
| - Hyperechoic foci |
| - Hyperechoic strands |
| - (Pseudo-) cysts |
| Rosemont Classification |
| Findings are categorized by giving different weight to each element in the diagnostic process of chronic pancreatitis. |
| Major criteria |
| A: - Hyperechoic foci of >2 mm with posterior accoustic shadow |
| - Lithiasis in main pancreatic duct |
| B: - Lobularity with honeycombing (3 or more contiguous lobules) |
| Minor criteria: |
| - Cysts |
| - Main duct dilation of more than 3.5 mm (1.5 mm at the tail) |
| - Irregular main duct contour |
| - Dilated side branches (>3) measuring >1 mm |
| - Hyperechoic main duct margins |
| - Stranding (hyperechoic lines >3 mm at least in 2 directions) |
| - Hyperechoic foci without posterior accoustic shadow (>2 mm) |
| - Lobularity without honeycombing |
| CrP diagnosis based on ultrasonographic criteria (from Sheel ARG et al, with minor modifications)74 |
| Standard criteria | Japanese criteria | Rosemont criteria |
|---|---|---|
| High probability | Definitive | Compatible |
| 5−9 criteria | Multiple calcifications in parenchyma | 1 major A ≥ 3 minor |
| Ductal calculi | 1 major A + 1 major B | |
| Ductal morphological changesa | 2 major A | |
| Early onset | Suggestive | |
| 3 EUS criteriab | 1 major A < 3 minorc | |
| 1 major B ≥ 3 minorc | ||
| ≥5 minorc | ||
| Indeterminate | Probable/possible | Indeterminate |
| 3–4 criteria | Signs suggestive of stones-plugs or dilatation of ductsd | 3–4 minorc |
| 1 major B ± <3 minorc | ||
| ≥2 minore | ||
| Normal | Normal | Normal |
| 0–2 criteria | 0 EUS criteria | <3 minor |
Irregular dilatation of main pancreatic duct and irregular dilatation of secondary ducts of variable intensity distributed thoughout the gland.
Hyperechoic foci without shadow, weft and lobularity with or without honeycomb structure. In addition, it must have more than 2 items from the following: recurent abdominal pain, raised levels of pancreatic enzymes in blood or urine, evidence of exocrine pancreatic insufficiency, alcohol intake over 80 g/d.
The diagnosis requires confirmation with other additional imaging techniques (ERCP, MRI, CT or pancreatic functional test).
Studies that have compared histopathology and EUS have not found a perfect correlation: With more than 5 criteria there was a high correlation with histology, but 50% of patients with less than 2 criteria had histological changes of CrP. Subtle alterations detected in EUS may disappear with time and have no significance.
An attempt was made to categorize these criteria by giving more relevance to some of them (Rosemont criteria),74 in particular lithiasis and lobularity of the parenchyma, but diagnostic precision has not improved.
Measurement of relative strain using elastography with or without SWV (shear wave velocity) measures offers objective data, has good diagnostic accuracy (91%)75 and correlate with the degree of fibrosis.76
HistologySCD-SCPanc 13. Histological examination of the pancreas should be a fundamental tool for diagnosis, but its pre-surgical use is limited by the difficulties in performing biopsies and by the patchy nature of the lesions.
SCD-SCPanc 14. The most required role of the pathologist in the study of chronic pancreatitis is to differentiate it from a malignant neoplasm, especially from ductal adenocarcinoma, and to identify autoimmune pancreatitis.
SCD-SCPanc 15. No consensus has been reached among pathologists to define which histological lesions are required to establish a diagnosis of chronic pancreatitis. Many would consider fibrosis and acinar atrophy acceptable and sufficient features for diagnostic purposes.
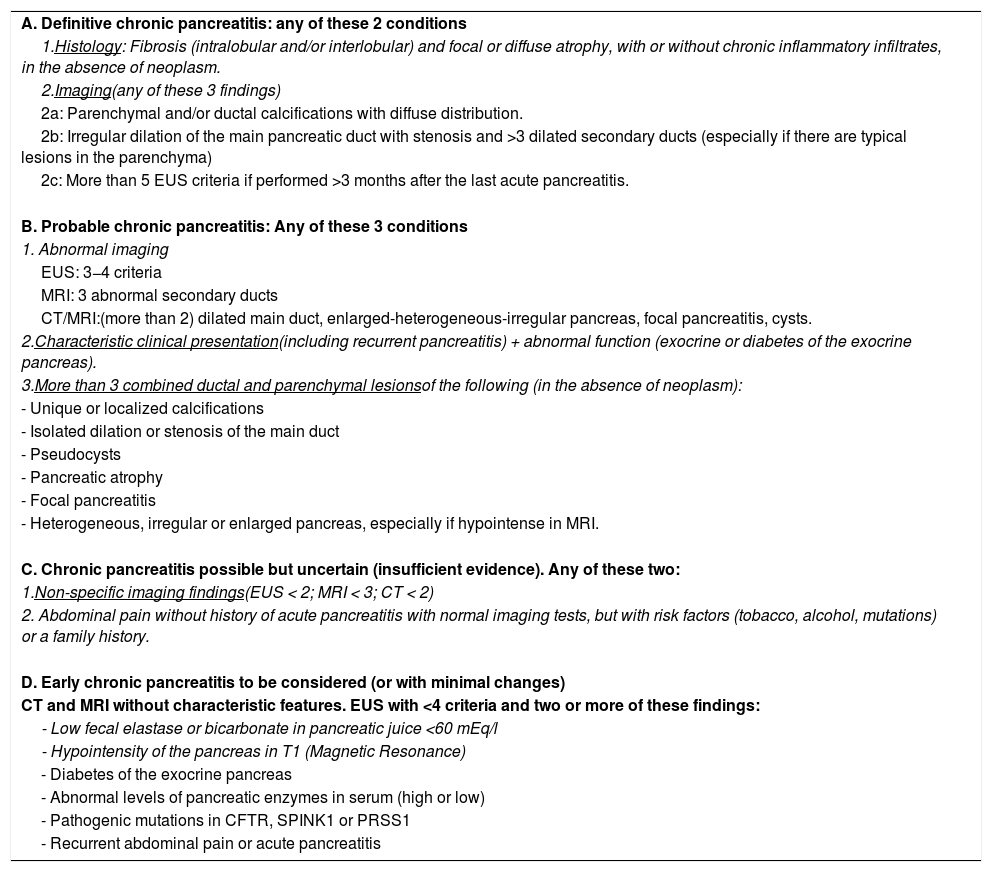
We support the diagnostic criteria described in Table 4.
Diagnostic criteria for chronic pancreatitis.
| A. Definitive chronic pancreatitis: any of these 2 conditions |
| 1.Histology: Fibrosis (intralobular and/or interlobular) and focal or diffuse atrophy, with or without chronic inflammatory infiltrates, in the absence of neoplasm. |
| 2.Imaging(any of these 3 findings) |
| 2a: Parenchymal and/or ductal calcifications with diffuse distribution. |
| 2b: Irregular dilation of the main pancreatic duct with stenosis and >3 dilated secondary ducts (especially if there are typical lesions in the parenchyma) |
| 2c: More than 5 EUS criteria if performed >3 months after the last acute pancreatitis. |
| B. Probable chronic pancreatitis: Any of these 3 conditions |
| 1. Abnormal imaging |
| EUS: 3−4 criteria |
| MRI: 3 abnormal secondary ducts |
| CT/MRI:(more than 2) dilated main duct, enlarged-heterogeneous-irregular pancreas, focal pancreatitis, cysts. |
| 2.Characteristic clinical presentation(including recurrent pancreatitis) + abnormal function (exocrine or diabetes of the exocrine pancreas). |
| 3.More than 3 combined ductal and parenchymal lesionsof the following (in the absence of neoplasm): |
| - Unique or localized calcifications |
| - Isolated dilation or stenosis of the main duct |
| - Pseudocysts |
| - Pancreatic atrophy |
| - Focal pancreatitis |
| - Heterogeneous, irregular or enlarged pancreas, especially if hypointense in MRI. |
| C. Chronic pancreatitis possible but uncertain (insufficient evidence). Any of these two: |
| 1.Non-specific imaging findings(EUS < 2; MRI < 3; CT < 2) |
| 2. Abdominal pain without history of acute pancreatitis with normal imaging tests, but with risk factors (tobacco, alcohol, mutations) or a family history. |
| D. Early chronic pancreatitis to be considered (or with minimal changes) |
| CT and MRI without characteristic features. EUS with <4 criteria and two or more of these findings: |
| - Low fecal elastase or bicarbonate in pancreatic juice <60 mEq/l |
| - Hypointensity of the pancreas in T1 (Magnetic Resonance) |
| - Diabetes of the exocrine pancreas |
| - Abnormal levels of pancreatic enzymes in serum (high or low) |
| - Pathogenic mutations in CFTR, SPINK1 or PRSS1 |
| - Recurrent abdominal pain or acute pancreatitis |
Abbreviations: EUS: endoscopic ultrasonography; MRI: Magnetic Resonance Imaging; CT: Computed Tomography.
Note: The exact percentage of asymptomatic patients is unknown. In the absence of abdominal pain, the combination of subtle morphological changes (hypointensity in T1), pathogenic mutations and low elastase may have diagnostic value.
CrP is characterized by diffuse or focal involvement in the form of fibrosis, parenchymal atrophy, ductal distortion and dilation, and a variable chronic inflammatory infiltrate67,77 containing lymphocytes, plasma cells and eosinophils, in addition to an acute component that includes neutrophils. Other characteristic features seen at pathological examination are lobular pattern, pseudocysts, intraductal stones, enlarged neural bundles, clustered islets, fibrous thickening and obliteration of blood vessels, and squamous metaplasia of the ductal epithelium.
Fibrosis can be perilobular or interlobular, and even intralobular. Islets are usually preserved.
Except for autoimmune pancreatitis (due to their own histological criteria) and groove pancreatitis (dilated ducts, cysts, microabscesses and fibrosis extending towards the duodenal wall), histological findings cannot discern the aetiology of CrP.
In general, diagnosis is well accomplished upon examination of a surgical specimen, but material is insufficient when obtained by cytological puncture, and it is suboptimal if a biopsy needle is used.76 If autoimmune pancreatitis is suspected, at least a pancreatic biopsy (not just cytology) is required for histological diagnosis confirmation.
Histological changes similar to CrP can be seen in elderly patients, in sections proximal to a ductal obstruction, in chronic renal failure, and most likely in smokers or long standing diabetic patients.
Pancreatic function (exocrine and endocrine)SCD-SCPanc 16. Exocrine function should be evaluated in all patients with CrP. We propose using faecal elastase determination as the reference test.
SCD-SCPanc 17. Patients must follow a program for early detection of diabetes by determining blood glucose and glycosylated haemoglobin at least once a year.
SCD-SCPanc 18. We consider the diagnosis of diabetes of the exocrine pancreas in the absence of typical features of type 1 or 2 diabetes and if associated with exocrine insufficiency and/or pathological pancreatic imaging tests.
Numerous tests have been described to diagnose EPI and grade its severity, but many have been abandoned for being considered invasive and cumbersome.78,79 Currently, the following can be determined:
- -
Pancreatic amylase, lipase or trypsin in serum. Low levels support a suspected diagnosis based on symptoms or imaging tests, with specificity close to 90%.80,81 Very low levels of all three enzymes indicate severe EPI.
- -
Bicarbonate in duodenal aspirate obtained by endoscopy after infusion of 0.2 µg of secretin. Repeated values below 80 mEq/L are diagnostic of EPI.67 Some American centres have adopted this test as a standard.
- -
Faecal elastase. Despite its limitations, we currently propose to use faecal elastase determination as the standard test. It is simple and allows serial testing. It has a sensitivity around 90–100% for severe and 63% for moderate insufficiency. A value below 200 mcg/g of faeces is considered abnormal, but false positives rates are a concern. Specificity improves at a cut-off level below 100, but sensitivity worsens. Values higher of 500 mcg/g exclude EPI. In case of previous pancreatic surgery, faecal elastase levels may not reflect accurately the degree of pancreatic functional derangement.56
- -
Fat absorption coefficient. It is difficult to perform: after a controlled diet (fats 100 g/day, 5 days) the stool is collected for 72 h and the amount of fat absorbed is determined, which must be >93%. It does not differentiate causes of steatorrhea, it is not available in many centres, and faecal manipulation is cumbersome. In case of previous pancreatic surgery, it is the test that best reflects exocrine insufficiency.56
- -
Mixed triglycerides breath test. Some claim to be more sensitive than faecal elastase and useful for optimizing enzyme treatment. But results are altered in liver disease, gastroparesis, bronchopathy or bacterial overgrowth, events frequently associated with CrP.
The value of exocrine function test in CrP assessment is highlighted in a 2013 study in which normal bicarbonate values in duodenal aspirate after secretin stimulation in patients with abdominal pain had a negative predictive value of 97% after a follow-up of 1–11 years.82
Probably the association of low elastase and low serum levels of enzymes has greater diagnostic accuracy for primary EPI, but studies supporting this association are lacking.
Diagnosis of diabetes of the exocrine pancreas can be made in patients who meet these conditions: 1- well-established diagnosis of diabetes mellitus; 2- exocrine pancreatic disease; and 3- reasonable evidence that diabetes is secondary to pancreatic disease. Of these 3 points, the third is the most controversial. Insulinopenia (demonstrated by low levels of C-peptide) is common and supports the diagnosis. However, insulin resistance may coexist favoured by PP deficiency and by intrapancreatic inflammatory events. Hypoglycaemic episodes are frequent due to associated glucagon deficiency.
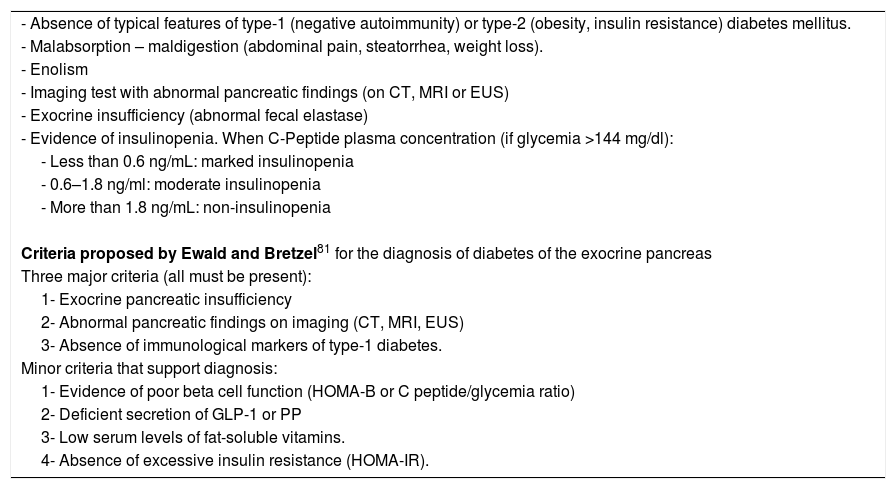
There are no standardized diagnostic criteria for “diabetes of the exocrine pancreas”, but the conditions detailed in Table 5 should help to suspect it. The diagnostic criteria of Ewald and Bretzel83 have not been validated.
Conditions suggesting diabetes of the exocrine pancreas.
| - Absence of typical features of type-1 (negative autoimmunity) or type-2 (obesity, insulin resistance) diabetes mellitus. |
| - Malabsorption – maldigestion (abdominal pain, steatorrhea, weight loss). |
| - Enolism |
| - Imaging test with abnormal pancreatic findings (on CT, MRI or EUS) |
| - Exocrine insufficiency (abnormal fecal elastase) |
| - Evidence of insulinopenia. When C-Peptide plasma concentration (if glycemia >144 mg/dl): |
| - Less than 0.6 ng/mL: marked insulinopenia |
| - 0.6–1.8 ng/ml: moderate insulinopenia |
| - More than 1.8 ng/mL: non-insulinopenia |
| Criteria proposed by Ewald and Bretzel81 for the diagnosis of diabetes of the exocrine pancreas |
| Three major criteria (all must be present): |
| 1- Exocrine pancreatic insufficiency |
| 2- Abnormal pancreatic findings on imaging (CT, MRI, EUS) |
| 3- Absence of immunological markers of type-1 diabetes. |
| Minor criteria that support diagnosis: |
| 1- Evidence of poor beta cell function (HOMA-B or C peptide/glycemia ratio) |
| 2- Deficient secretion of GLP-1 or PP |
| 3- Low serum levels of fat-soluble vitamins. |
| 4- Absence of excessive insulin resistance (HOMA-IR). |
Abbreviations: EUS: endoscopic ultrasonography; MRI: Magnetic Resonance Imaging; CT: Computed Tomography; HOMA: Homeostatic Model Assessment; PP: pancreatic polypeptide.
To differentiate diabetes of the exocrine pancreas from type 1 diabetes (especially in young patients) it is useful to check for the absence of immunological markers, such as anti-insulin, anti-GAD65, anti-IA-2 or anti-pancreatic islet antibodies.
HOMA (after Homeostatic Model Assessment) in its linear form has two variants: HOMA-IR (assesses insulin resistance) and HOMA-B (assesses beta cell function).
HOMA-IR: Fasting insulin (mIU/L) × fasting blood glucose (mmol/L)/22.5
Fasting insulin (mIU/L) × fasting blood glucose (mg/dL)/405
Normal <1.96. Insulin resistance should be suspected when value between 1.96 and 3.
Insulin resistance if value >3
HOMA-B: 20 × Fasting insulin (mIU/L)/(Fasting blood glucose (mmol/L) − 3.5) × Fasting insulin (mIU/L)/(Fasting blood glucose (mg/dL) − 63)
Normal values depend on the population studied. Values below the lower quartile of the healthy reference population (75%) suggest poor beta cell function.
Glycaemia mmol/L = glycaemia mg/dl × 0.0555
HOMA2 takes into account the previous equations and gives them a non-linear value relative to the healthy young population to which a value of 100% is assigned. A HOMA2 calculator from the University of Oxford can be downloaded here:
https://www.dtu.ox.ac.uk/homacalculator/.
It must be taken into account that the HOMA parameters only guide towards insulin resistance and towards the function of beta cells in basal conditions. But beta cells have a dynamic behaviour that responds to stimuli. In the presence of normal glycaemia, it is difficult to assess basal insulin deficiency. On the other hand, in patients on insulin treatment, the HOMA-B is more correctly calculated with C-peptide values using the Oxford University calculator.
An additional criticism of the Ewald and Bretzel criteria is that they require the presence of exocrine insufficiency (EPI), but diabetes of the exocrine pancreas can appear in the absence of apparent EPI.
Diagnostic criteriaSCD-SCPanc 19. The panel of specialists in the present document proposes a combined adaptation of the diagnostic criteria for chronic pancreatitis from the American and Japanese Pancreatic Societies. The final goal is to establish the diagnosis with degrees of evidence and, if diagnosis is confirmed, to define the exocrine and endocrine functionality.
SCD-SCPanc 20. Incipient or early CrP does not have a precise definition or reliable diagnostic criteria in the absence of histological data. Early CrP could be viewed as an early stage of the disease with minimal or absent radiological or ultrasound changes.
The diagnosis of CrP is simple in advanced forms, but difficult in initial stages. Diagnosis is based on demonstration of characteristic histological lesions, abnormalities in imaging tests, and functional derangement. Accurate diagnosis is very important due to therapeutic and prognostic implications. On the other hand, the ability to exclude a CrP with confidence is essential in the differential diagnosis of abdominal pain, pancreatic mass or cyst, chronic diarrhoea or diabetes.
In order to standardize the diagnosis of CrP some societies have proposed diagnostic criteria that provide variable reliable evidence. We propose the criteria detailed in Table 467,84 taking into account the following statements:
- 1
Criteria for definitive CrP are valid in the absence of neoplasia, and do not require categorizing patient's symptoms.
- 2
When EUS is used, lesions suggestive of CrP can be identified during months following acute pancreatitis, in the elderly, in smokers, and in diabetics.
- 3
In the acquisition of images, specific protocols for the pancreas must be followed.
- 4
To use the parameter “abnormal pancreatic function” as a diagnostic item we should note that faecal elastase values are valid if determined at least 4 months after acute pancreatitis, that maximum bicarbonate excretion <60 mEq/L in the endoscopic pancreatic function test has high diagnostic accuracy for EPI, and that diagnosis of diabetes of the exocrine pancreas must be unquestionable.
- 5
Patients with “chronic pancreatitis without sufficient evidence” should adhere to a follow-up program to reject or reconsider the diagnosis.
- 1
These criteria should help us to establish a definitive or probable diagnosis of CrP, or to conclude that there is not enough evidence to maintain a diagnosis with some confidence.
Early chronic pancreatitis will be discussed in greater detail in Part 3 of this series.
Evaluation of pancreatic painSCD-SCPanc 21. It is important to characterize the pancreatic pain in each patient and register pain intensity.
One-dimensional (numerical scale from 1 to 10, visual analogue scale) and multidimensional (Izbicki Pain Score, Brief Pain Inventory, McGill Pain Questionnaire) scales can be used.85 Pain can also be studied and quantitated using some other tests that are restricted to specific centres or for research purposes. The quantitative sensory testing (QST) study aim at differentiating the central sensitization component of chronic pancreatic pain and thus provide a better guide for treatment.85,86
StagingSCD-SCPanc 22. We propose to categorize the disease according to the recommendations of the American Pancreatic Association with minor modifications.
To categorize CrP, we should describe the suspected causal factors, the morphological stage by imaging tests (from I to IV) and the functional stage (from A to X) based on the Cambridge classification (Table 6).
Staging of chronic pancreatitis.
| It requires to specify the associated etiological factors as active or inactive (A), grade pancreatic morphology on imaging tests (B) and estimate current functional stage (C). |
| A- Associated active/inactive etiological factors. |
| B- Morphological staging: Equivocal, mild, moderate, marked |
| Equivocal (I) | Mild (II) | Moderate (III) | Marked (IV) | |
|---|---|---|---|---|
| CT/MRI | 1 of the following:- Enlarged main duct (2–4 mm)- slight enlarged pancreas- heterogeneous parenchyma- collections <10 mm- irregular ducts- focal pancreatitis- increased main duct wall echogenicity- irregular pancreas contour | Diffuse hypointensity in T1or≥2 features of those described for equivocal CrP | Same as mild | Same as moderate + ≥1 of- collections >10 mm- enlarged pancreas- diffuse intraductal or parenchymal calculi- duct obstruction- Strictures or gross irregularity |
| EUS (Criteria 0−9) | 0−2 | 3–4 | ≥5 | |
| Ductal Morphology (better with secretin-MRI) | <3 abnormal side-branch changes | ≥3 abnormal side-branch changes | >3 abnormal side-branch changes and abnormal main duct | Moderate changes + 1 of the following:- obstruction- obstruction- Marked irregularity or dilation of the main duct |
| C- Functional stage |
| A- Normal |
| B- Exocrine secretory dysfunction (abnormal endoscopic secretin stimulation test) |
| C- Exocrine secretory dysfunction (abnormal endoscopic secretin stimulation test) |
| D- Exocrine insufficiency (low fecal elastase, low serum trypsin, steatorrhea) |
| E- C + D |
| F- Unknown |
| Staging examples: |
| Patient is active smoker patient with stage IV E chronic pancreatitis |
| Patient with a pathogenic CFTR mutation with mild chronic pancreatitis without functional impairment (Stage II A) |
| Patient is ex-enolic with moderate chronic pancreatitis and endocrine insufficiency (Stage III D) |
There are no prospective studies validating the different classifications published on evolutionary stages. The MANNHEIM, Büchler and the American Pancreatic Association classifications may provide more descriptive information.67,87,88 In any case, advanced stages do not necessarily associate to more serious clinical conditions, but they are usually related to a greater number of complications.
There seems to be a good concordance when CT is compared with MRI89 in terms of categorizing patients according to the Cambridge classification.
We support the American Pancreatic Association recommendations to characterize patients according to the degree of diagnostic evidence, the associated etiological factors, the imaging stage and the functional competence. For example, we should be able to conclude that an active smoker has definitive stage IV E chronic pancreatitis (i.e., with exocrine and endocrine insufficiency). Or that another patient has probable chronic pancreatitis carrying the N34S mutation in SPINK1, with inactive enolism and preserved pancreatic function (stage II A).
Moreover, from a histological point of view, there is no universally accepted staging system. Damage can be categorized as mild, moderate and severe, but this methodology has not been validated77.
Guidelines to perform genetic assessmentSCD-SCPanc 23. We recommend to perform genetic studies to all patients with CrP who:
- -
are of paediatric age.
- -
onset of the disease <35 years of age.
- -
have first- or second-degree relatives with pancreatic pathology related to CrP (pancreatitis-diabetes-cancer).
Genetic assessment would also be acceptable in patients:
- -
with acute recurrent or chronic pancreatitis of uncertain aetiology.
- -
with characteristic symptoms of cystic fibrosis (bronchiectasis, infertility, liver disease).
- -
in direct relatives of patients with CrP who carry pathogenic mutations.
In the rest of patients with CrP, performance of genetic studies will depend on medical criteria, availability of resources and acceptance of the patient.
Genetic studies should be followed by family counselling in affected individuals.
Differential diagnosisSCD-SCPanc 24. CrP should be differentiated from other causes of abdominal pain, chronic diarrhoea, malnutrition, pancreatic duct dilation, pancreatic cysts and pancreatic insufficiency of another aetiology. Idiopathic hyperamylasemia and pancreatic cancer should also be considered.
Special care must be taken when some situations coincide, such as abdominal pain of unclear cause with pancreatic cysts or idiopathic hyperamylasemia.
Some differential diagnosis worth to be discussed further:
CrP vs. other EPI causes (Table 7)SCD-SCPanc 25. CrP is the leading cause of EPI in adults. Other common causes are pancreatic neoplasms, any obstruction of the pancreatic duct, previous resections and cystic fibrosis.
Causes of exocrine pancreatic insufficiency.
| Primary causes |
|
|
|
|
|
|
|
|
| Secondary causes |
|
|
|
|
| Uncertain origin (possibly multifactorial) |
|
|
|
|
|
It also appears after gastrectomies and resections of the small intestine due to impairment of secretin and cholecystokinin secretion and postcibal asynchrony. It has been described in 12–50% of long-standing diabetic patients without signs of chronic pancreatitis. Some elderly patients develop marked pancreatic atrophy or fatty degeneration without other signs of CrP.
SCD-SCPanc 26. During a period of time following acute oedematous (12%) or necrotizing (90%) pancreatitis there is transient EPI of variable degree, which correlates with the extent of necrosis. It can become permanent in severe cases.
Moreover, extensive necrotizing pancreatitis can lead to permanent EPI without any other evidence of CrP.90
Recurrent pancreatitis vs. chronic pancreatitisSCD-SCPanc 27. After repeated attacks of acute pancreatitis, and depending on the severity of the episodes, parenchymal or ductal fibrotic changes may persist without yet being considered a form of CrP. On the other hand, 10–30% of patients with acute pancreatitis will develop RAP.
Recurrent acute pancreatitis (RAP) represents a very relevant clinical problem.91 CrP can present with episodes of acute pancreatitis.2 The subgroup of patients with RAP that will progress to chronic pancreatitis is 13–36% being alcohol and tobacco consumption distinct risk factors.1,2,18 RAP is also a characteristic feature in patients with CFTR, PRSS1, SPINK1, or CTRC pathogenic mutations, which are present in up to 58% of children with RAP.92
Imaging tests should be performed in these individuals to look for morphological changes that often only become patent during follow-up. When it comes to RAP, EUS is superior to MRI in identifying biliary pathology, periampullary lesions, or CrP,93 but care must be taken not to over diagnose CrP.
Pseudocyst or duct dilation vs. cystic neoplasiaSCD-SCPanc 28. Intraductal Papillary Mucinous Neoplasia (IPMN) and CrP share clinical and morphological features and sometimes do coexist. Development of IPMN in a pancreas with CrP is not uncommon.
SCD-SCPanc 29. Identification of papillary structures in the main duct by pancreatoscopy confirms IPMN. Identification of activating mutations (R201H and R201C) in the GNAS oncogene in fluid aspirates subscribes the presence of IPMN with specificity close to 100% and sensibility of 40–60%.
Primary, secondary, or mixed duct IPMN may induce morphological changes consistent with CrP: dilation of the main duct and/or secondary ducts, glandular atrophy and calcifications. When IPMN presents episodes of RAP the differential diagnosis with CrP is difficult. On the other hand, secondary duct IPMN and mucinous cystadenoma can also cause RAP and be mistaken for pseudocysts.
The presence of one or multiple pancreatic cysts and/or the dilation of the main duct, sometimes with intraductal or parenchymal calcifications, can be indistinguishable from CrP. In addition, malignant IPMN may be associated with parenchymal lesions typical of CrP (detected by EUS), evidence of EPI, and low serum levels of pancreatic enzymes, all characteristic features of CrP. The differential diagnosis requires imaging tests (CT, MRI, EUS, pancreatoscopy)94 and the analysis of pancreatic fluid obtained by EUS-guided puncture or by cannulation of the papilla. The diagnosis of IPMN is supported by the finding of mucin or mucinous epithelial cells (although this finding is neither sensitive nor specific), and CEA levels greater than 192 ng/mL. In these cases, identification of mutations in GNAS (100% specific for mucinous neoplasms) and KRAS genes (highly specific for mucinous neoplasms, although they can also be present in chronic pancreatitis) is of great value.
Detection of ductal communication of the cyst, or homogeneous dilation of the main duct without stenosis suggests IPMN, but does not exclude coexistence of CrP. Currently the two approaches with the best diagnostic specificity to detect IPMN are pancreatoscopy and the analysis of GNAS in pancreatic fluid.95,96
Inflammatory mass vs. pancreatic cancerSCD-SCPanc 30. Groove pancreatitis, AIP and any inflammatory mass associated with CrP must be differentiated from pancreatic cancer.
CrP itself carries a high risk of developing pancreatic cancer. We should use a combined battery of imaging tests and, in any doubt, obtain samples for cyto-histological examination whenever possible. EUS with fine needle aspiration or biopsy can help the diagnosis if it is clearly positive, but negative biopsies have to be interpreted with caution. If diagnostic doubts persist, we should not hesitate to repeat imaging tests, newer biopsies, or even consider diagnostic laparotomy.
Pancreatic cancer tends to be hypodense and hypovascular and is usually associated with proximal pancreatic atrophy. A double duct sign (both common bile duct and pancreatic duct dilation with collapse at the level of the mass) can be detected. Vascular invasion, involvement of neighbouring tissues, or metastases are pathognomonic.
The finding of pancreatic duct dilation >11.5 mm and common bile duct >14.5 mm has been suggested as a cancer marker in patients with CrP.97 The penetrating duct sign (dilated duct that progressively narrows into the mass) is present in 85% of inflammatory masses but in just 4% of neoplastic ones.98
Other features that help differential diagnosis (but none are pathognomonic) are the shape of ductal strictures, morphology of the mass including displacement of calcifications, vascular involvement, value of the apparent diffusion coefficient (ADC) in MRI, MM parameter in Resonance DWI, FDG uptake in PET/CT and perfusion imaging parameters.99
Multidetector CT can differentiate between CrP and cancer with a sensitivity of 89% and a specificity of 70%100 versus 96% and 91% for contrast-enhanced EUS modalities (CEHMI-EUS).
Features that suggest a benign mass by EUS include a homogeneous pattern with hyperechoic septa, calcifications, cysts, multilobular appearance, and positive Doppler signals. Cancer is suggested by a hypoechoic mass, periductal hypoechogeneity, and displacement of calcifications.101 The sensitivity and specificity of isolated EUS to differentiate cancer from CrP is only 64% and 75%, respectively.
Cytology, elastography, and contrast infusion are useful tools.102 The diagnostic yield of EUS-guided cytology or biopsy in pancreatic masses varies from 80 to 95%,103 but in the presence of CrP the yield decreases to 50–75%.104
It is particularly difficult to differentiate AIP from cancer.105,106 Accuracy can be increased to 90% with >7 punctures using a larger needle and with in situ evaluation by an expert cytopathologist.107
EUS elastography can differentiate benign from malignant masses with a sensitivity of 88–93% and specificity of 66–83%. Using contrast-enhanced EUS pancreatic cancer appears hypovascular with delayed and diminished uptake as compared to an inflammatory mass. It achieves sensitivities and specificities close to 95%.
CA 19.9 is the only serum marker currently used in clinical settings. It has a sensitivity of 77% and a specificity of 86%, but the sensitivity drops to <50% in small neoplasms and has 23% of false positives.
Chronic pancreatitis vs. liver cirrhosisSCD-SCPanc 31. Differential diagnosis is important because ascites from pancreatic origin usually does not respond to diuretic treatment or repeated paracentesis (of a hyperproteic fluid) that will only increase hypoproteinemia and may accelerate an unfavourable course.
Patients with CrP and ascites are easily diagnosed with liver cirrhosis because they can have an enolic habit with steatosis and hypertransaminemia, have oesophageal varices (secondary to splenic or portal thrombosis), splenomegaly, hypoalbuminemia and a prolonged prothrombin time (due to malnutrition). In addition, many patients have concomitant chronic liver disease. Because pancreatic ascites has elevated white blood cells, these patients are often diagnosed with spontaneous bacterial peritonitis.
Pancreatic ascites usually has high concentration of amylase and proteins. It is also useful to detect a serum albumin to ascites gradient <1.1, or an ascites/serum albumin ratio >0.59. In patients with advanced CrP and marked pancreatic atrophy, amylase in ascites fluid may be low. When diagnosis is unclear due to coexisting liver disease hepatic hemodynamics should be performed, because if the portal pressure gradient is <10 mmHg an hepatic origin of the ascites is unlikely.
Conflict of interestXavier Molero is consultant investigator for Amadix Advanced Marker Discovery S.L (Acera de Recoletos 2, 1B, 47004 Valladolid) in the research project on pancreatic cancer AMD-CPA-2016-01
Xavier Molero has obtained a research grant on fundamental investigation on cystic fibrosis in mice from Vertex Pharmaceutical Inc., 50 Northern Avenue, Boston, MA 02210, USA.
None of the other above authors declares any other conflict of interest.
Please cite this article as: Molero X, Ayuso JR, Balsells J, Boadas J, Busquets J, Casteràs A, et al. Chronic pancreatitis for the clinician. Part 1: Etiology and diagnosis. Interdisciplinary position paper of the Societat Catalana de Digestologia and the Societat Catalana de Pàncrees. Gastroenterol Hepatol. 2022;45:231–248.