Gastritis cystica profunda (GCP) is a rare hyperplastic lesion with unclear pathogenesis histologically characterized by the presence of gastric glands in the submucosa and even muscularis propria of the stomach with normal overlying mucosa.1,2 There are two histological patterns of gastritis cystica poliposa: gastritis cystica superficialis, in which cystic glands are limited to the mucosal layer; and GCP in which the cystic lesion locates within the submucosa and muscularis propria.3 Clinical manifestations of GCP are variable and can include gastrointestinal bleeding, epigastric pain and weight loss.1 An unspecified mucosal insult or injury is widely accepted as etiological mechanism but the pathophysiology is unknown.1–4 We present a case of acute gastrointestinal bleeding caused by gastritis cystica profunda mimicking a gastrointestinal stromal tumor in a patient without previous gastric surgery.
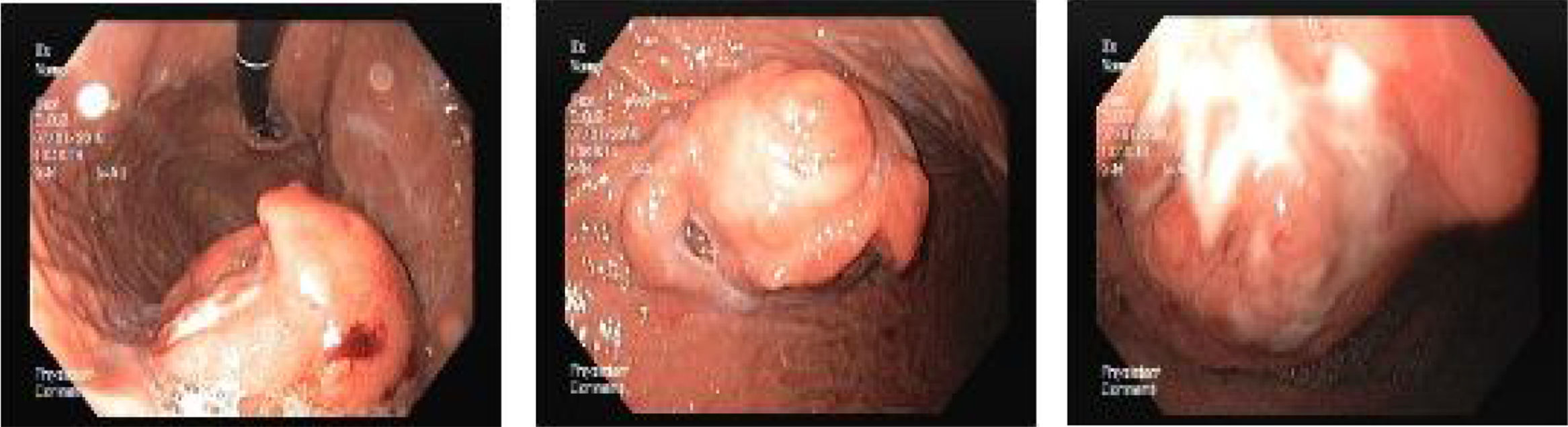
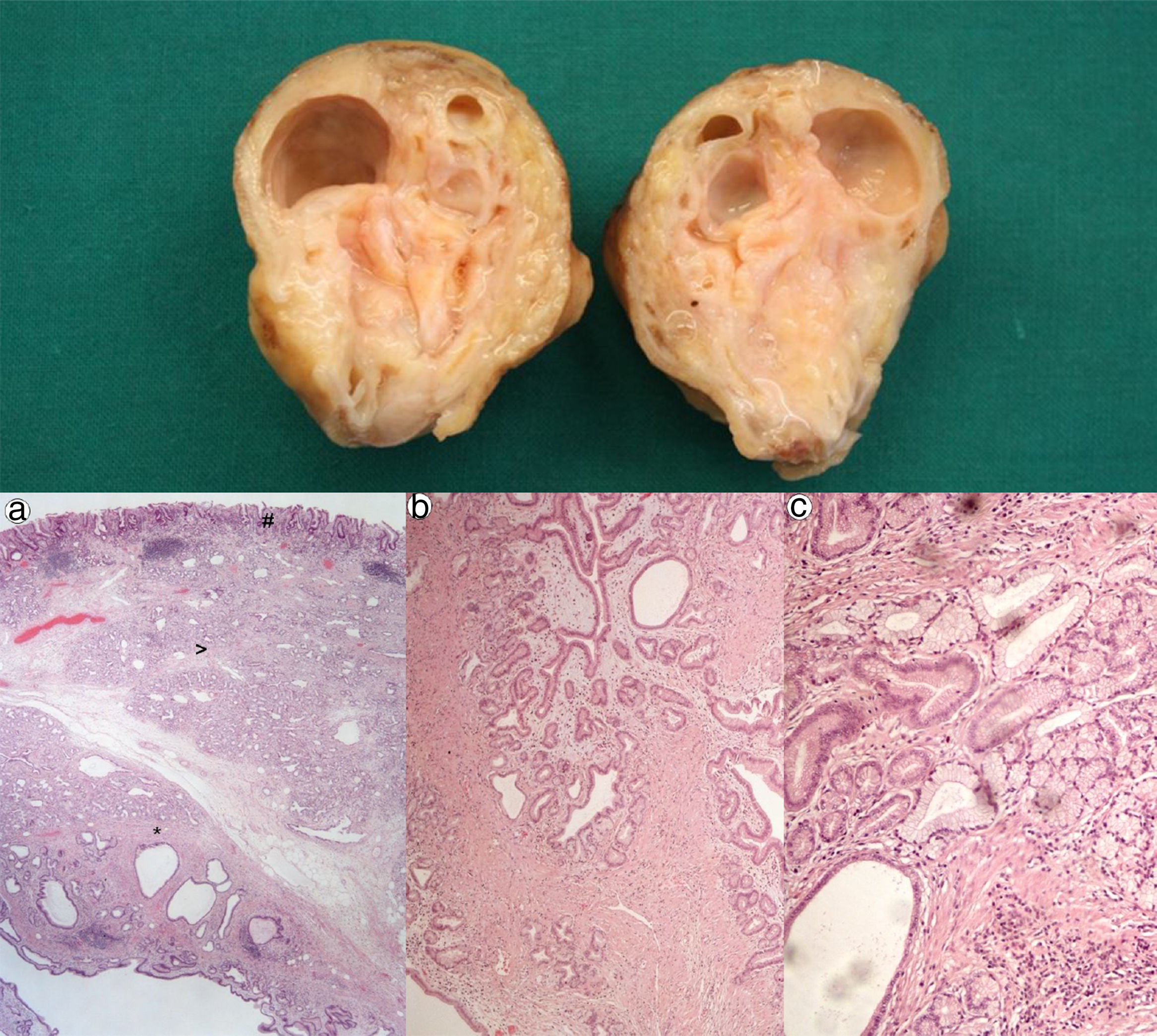
A 67 year-old man was admitted for melena during 4 days associated with syncope. He had a previous history of atrial fibrillation and was medicated with warfarin. At admission, he was hemodynamically stable and had no abdominal pain. Laboratory tests revealed normocytic anemia with hemoglobin 8.8g/dl (previous value: 13.5g/dl) and an INR of 2.3. Upper gastrointestinal endoscopy (UGIE) revealed a 40mm polypoid lesion in the gastric body with normal mucosa surface and a central 15mm ulcerated bleeding as which was very suggestive of a gastrointestinal stromal tumor (GIST) (Fig. 1). There were no esophageal or duodenal visible bleeding lesions. Biopsies performed during UGIE revealed aspects of chronic active gastritis and Helicobacter pylori was identified. Upper endoscopic ultrasonography (EUS) showed 40mm submucosal a hypoechogenic and heterogeneous mass with cystic areas and no perilesional adenopathies. The EUS findings could also correspond to a GIST. EUS guided fine-needle aspiration (FNA) using 19 gauge needle was preformed but the sample was insufficient for evaluation. A full body computed tomography was performed and revealed a 4×6cm gastric intraluminal lesion without signs of invasion or metastatic disease. The diagnosis of a gastric GIST was assumed and the patient was proposed to surgical resection of the lesion. Macroscopically, a polypoid lesion with a nodular surface and a central ulcer was observed, with multiple cysts and solid areas on cross-section (Fig. 2). Histologically (Fig. 2), the gastric mucosa showed focal lesions of chronic atrophic gastritis with activity, hemorrhage and ulceration, the submucosa and muscular propria displayed an abundant cystically dilated pyloric-type and foveolar-type glandular proliferation, without mitoses or atypia; surrounding the glands there was a thin layer of lamina propria and fibromuscular hyperplasia. The diagnosis of Gastritis cystica profunda was made. Surgical margins were free of lesion.
Cross-section shows multiple cysts with diameters between 0.2 and 1.5cm, filled with transparent and mucinous-looking liquid. (a) (H&E ×20): gastric wall including mucosa (#), submucosa (>) and muscularis propria (*) with abundant cystic glandular proliferation; (b) (H&E ×40): surrounding the glands there is a thin layer of lamina propria and fibromuscular hyperplasia; (c) (H&E ×100): the glands are lined by pyloric-type and foveolar-type epithelium, without mitoses or atypia.
In the majority of reported cases, GCP occurs in patients with a history of gastric surgery, in particular Billroth II procedure.2,3 It is unclear if it is secondary to chronic inflammation as consequence of duodenal reflux, foreign body reaction or ischemic injury as a result of the surgery.1,2,5,6 Nevertheless, the interruption of the muscularis mucosae appears to allow migration of epithelial cells into the submucosal layer and subsequent cystic dilation.2,7 In the unoperated stomach, the cause may be congenital in origin in patients with no prior gastric ulceration or trauma history.3 It is more common in men and most frequently develops in the gastric body,3 as seen in this case. Our patient had a history of chronic atrophic gastritis with H. pylori infection which in this case can be considered as a possible etiological factor. The presentation symptoms are not specific and, endoscopically it is impossible to differentiate from other entities like polyps or GIST.2 As biopsy samples are restricted to the spared mucosal the results are often not diagnostic.3,8 CT can show a heterogeneously iso- to hypoattenuating intraluminal lesion with multiple small cysts but the appearance may be similar to GIST.3,9 On EUS, GCP can appear as a polymorphic, homogeneous cystic mass with a minimal solid component within the gastric mucosae which is also not specific of GCP.1,3 However, the accuracy of EUS-FNA with immunostaining in preoperative GIST, diagnosis has been reported at 91%–100%.10 Some patients might have to undergo gastric resection when it is impossible to make a definite diagnosis with radiologic study or endoscopic biopsy, as seen in this case. Case reports have revealed the possibility of a malignant transformation from GCP, even in an unoperated stomach, but the incidence of malignancy in GCP patients remains unknown.3 Epstein–Barr virus might have a role as a premalignant factor in cancer tissue with GCP.10 Given the lack of a pathognomonic endoscopic or radiographic appearance of GCP, diagnostic and surveillance guidelines are not available and further studies are required.
FundingNo funding.
Conflicts of interestNo conflicts of interest.