Adenocarcinoma of the anal canal is a very uncommon neoplasm which accounts for 5% of all anorectal neoplasms and 1.5% of gastrointestinal tumours.1,2 According to the World Health Organization, there are 3 distinct types. The first originates in the transitional mucosa of the superior canal. The second derives from the anal glands (duct). The third derives from a chronic perianal fistula. Patients with tumours of the anal canal, regardless of their type, present a higher percentage of advanced disease and distant metastasis as well as lower overall survival compared to patients with rectal squamous carcinoma. Given the limited number of cases published, no treatment regimen has been completely confirmed3,4; however, most authors advocate for a treatment with neoadjuvant chemotherapy followed by radical surgery.5 Below we report an extremely rare case of perianal eccrine hydroadenocarcinoma in a patient previously diagnosed with Crohn's disease.
A 42-year-old man had a history of Crohn's disease for the past 20 years, multiple chronic perianal fistulisations and derivative colostomy in 2006. The patient was referred to surgery for biopsy of painful inguinal lymphadenopathy and perianal Tru-Cut biopsy to rule out degeneration of a chronic perianal fistula. The pathology study showed that the architecture of the lymph node had been completely replaced by an atypical cell proliferation of epithelial origin organised in the form of solid nests and adopting a ductal pattern with focal cribiform morphology in other areas. The neoplastic cells were large in size, featuring pleomorphic nuclei with granular chromatin, obvious nucleoli and abundant mitosis. The cytoplasm varied (pale eosinophilic, granular or with a change towards clear-cell). The cell immunophenotype was positive for p53 (80%), CK8, CK19, epithelial membrane antigen (EMA), carcinoembryonic antigen (CEA), CKAE1/AE3 and CAM 5.2 with negative immunoexpression of 34BE12, calretinin, CD30, CD117, vimentin, a-inhibin, CK7, p504S, p63, PSA, CDX2, CK20, CD10 and S100.6 EMA, CK8, CK19 and CEA are usually present in the eccrine and apocrine glands. The morphology and immunophenotype reported matched the findings of the Tru-Cut biopsy, and a diagnosis was made of lymph node metastasis of hydroadenocarcinoma deriving from perianal eccrine glands.
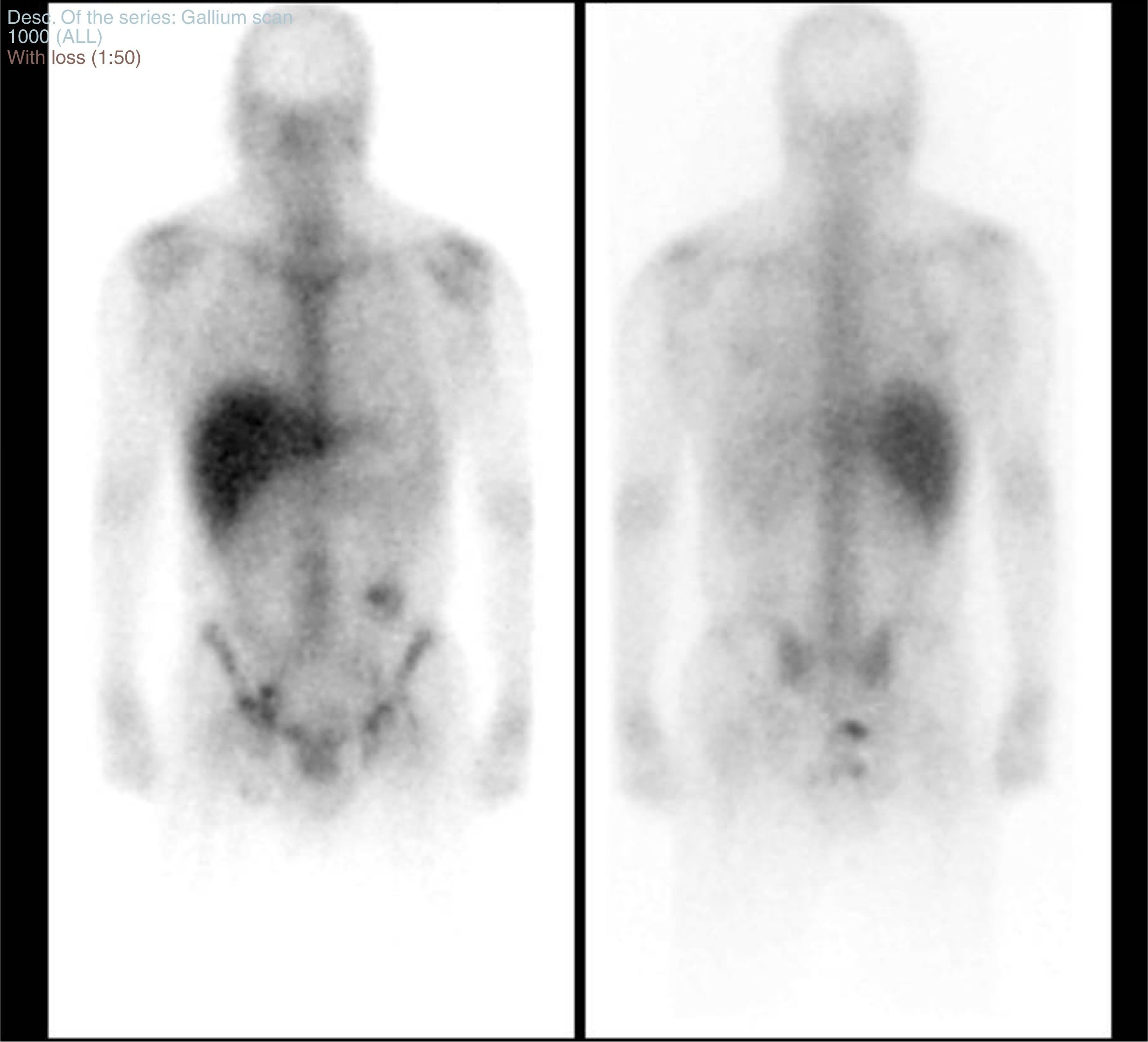
An extension study was performed through a CT scan of the chest and abdomen, pelvic MRI and a body scan with gallium citrate-67 (Fig. 1). The study with gallium citrate-67 revealed a pathological deposit of activity in the perianal region consistent with an inflammatory/septic condition.
Body scan obtaining planar images in anterior and posterior projection following intravenous administration of gallium citrate-67.
A pathological deposit of activity in the perianal and rectal region consistent with an inflammatory/septic condition was detected. In the left inguinal region there was a small deposit of moderate intensity suggesting reactive lymphadenopathy in that location. No other deposits were seen in the abdomen or in the rest of the body scan suggesting lymphadenopathic lesions and/or infectious conditions.
There was increased activity in the region of the colostomy probably due to a physiological deposit of gallium as it was eliminated through the bowel.
The CT scan showed extensive lymphadenopathy in the internal and external iliac lymph nodes and the bilateral inguinal lymph nodes. Findings of complex perianal disease with multiple fistula tracks and abscessifications were detected. In addition, MRI identified a large heterogeneous mass with an infiltrative appearance involving tissue from the most caudal segment of the rectum, pelvic floor musculature, right gluteus maximus and even the subcutaneous tissue and skin.
With a diagnosis of infiltrative neoplastic impairment of the anal canal (T4) with spread to the lymph nodes, the case was discussed in the multidisciplinary committee. Following a review of the literature, a decision was made to treat with neoadjuvant chemotherapy.7 A total of 12 cycles with a FOLFOX (folinic acid, fluorouracil and oxaliplatin) regimen and 8 more cycles without oxaliplatin (with 5-fluorouracil) were administered.
The patient was re-evaluated by means of MRI of the abdomen and pelvis. This confirmed tumour progression associated with complex fistulas and perilesional abscessification, and identified progressive inguinal and external iliac lymphadenopathy. The extension study (CT scan) showed no signs of disseminated disease.
Surgery was planned and a local resection was performed in which an ulcerated and gangrenous perianal neoplasm 8cm in diameter with a base measuring 4cm was resected until macroscopically clear margins were achieved. The patient's clinical course was favourable, with hospital discharge on the second day of the post-operative period.
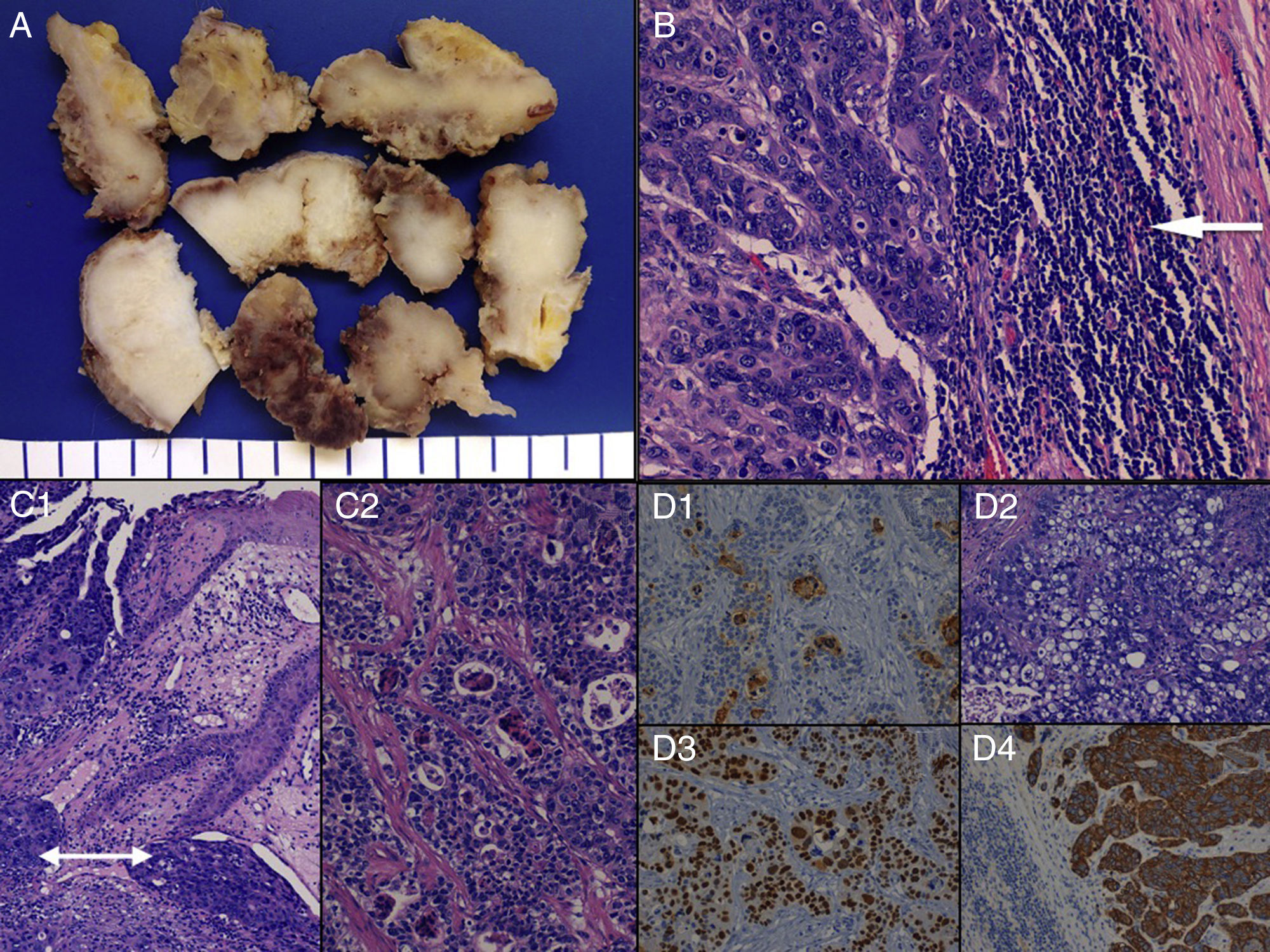
The pathology study of the surgical specimen (Fig. 2) showed a malignant, invasive primary epithelial neoplasm which could be categorised as an eccrine hydroadenocarcinoma with cutaneous ulceration, focal necrosis and a mixed morphological pattern (solid, cribiform, glandular and clear-cell changes) with sphincter muscle infiltration. The patient is currently in follow-up with no local recurrences or distant disease.
Macroscopic (A) and microscopic (B-D) characteristics of eccrine hydroadenocarcinoma. (A) Perianal resection specimen sections consisting of a whitish, homogeneous, compact tumour. (B) Microscopic image of lymph node metastasis due to hydroadenocarcinoma (see tumoural epithelial nests to the left and preserved rim of peripheral lymphoid tissue [arrow] to the right) (H&E× 400). (C) Infiltration, perianal cutaneous tumoural ulceration and lymphatic vascular invasion (double arrow) (H&E× 250) (C1), and microscopic image of the cribiform pattern of the neoplasm (H&E× 250) (C2). (D) Immunoexpression in eccrine gland lumina of carcinoembryonic antigen (CEA× 200) (D1); tumoural areas with clear-cell change (H&E× 200) (D2); intense, diffuse positivity for p53 (p53× 200) (D3); and a neoplastic component with positive immunostaining for cytokeratin 8 (CK8× 200) (D4).
Eccrine hydroadenocarcinoma is a rare tumour deriving from the eccrine sweat gland. It was reported by Stout and Cooley in 1951.8 It has malignant behaviour with slow, locally aggressive growth (high recurrence), with potential for metastasis through regional lymph nodes to the perioesophageal, peribronchial, periaortic and retroperitoneal lymph nodes and through the bloodstream to the bones, vertebrae, ribs, pelvis, lungs and pleura.9 The first major study related to its classification was published in 1968 by Berg and McDivitt.10 It may be identified in the literature by other names such as clear cell hydroadenocarcinoma, malignant clear cell hydroadenoma, solid-cystic adenocarcinoma, malignant acrospiroma, malignant clear cell myoepithelioma and eccrine clear cell carcinoma. We conducted a systematic review of the literature and found only 2 cases in the perianal region reported to date.6,9
It may be difficult to distinguish from adenocarcinoma of the lower rectum having spread to the anal canal. It is crucial that it be suspected early to prevent delayed diagnosis and treatment, and it should be taken into account in cases of recurrent perianal fistulas and perianal abscessifications, especially in patients with fistulising Crohn's disease. Although there is no standardised protocol, the most current literature classifies it as an adenocarcinoma of the anal canal, subject to neoadjuvant treatment followed by radical surgery with subsequent adjuvant treatment to prevent micrometastases.4
Please cite this article as: Pareja-López Á, Ferrer-Márquez M, Berenguel-Ibáñez MM, Espínola-Cortés N, Velasco-Albendea FJ. Hidroadenocarcinoma ecrino perianal en el contexto de una enfermedad de Crohn fistulizante. Gastroenterol Hepatol. 2018;41:450–452.






![Macroscopic (A) and microscopic (B-D) characteristics of eccrine hydroadenocarcinoma. (A) Perianal resection specimen sections consisting of a whitish, homogeneous, compact tumour. (B) Microscopic image of lymph node metastasis due to hydroadenocarcinoma (see tumoural epithelial nests to the left and preserved rim of peripheral lymphoid tissue [arrow] to the right) (H&E× 400). (C) Infiltration, perianal cutaneous tumoural ulceration and lymphatic vascular invasion (double arrow) (H&E× 250) (C1), and microscopic image of the cribiform pattern of the neoplasm (H&E× 250) (C2). (D) Immunoexpression in eccrine gland lumina of carcinoembryonic antigen (CEA× 200) (D1); tumoural areas with clear-cell change (H&E× 200) (D2); intense, diffuse positivity for p53 (p53× 200) (D3); and a neoplastic component with positive immunostaining for cytokeratin 8 (CK8× 200) (D4). Macroscopic (A) and microscopic (B-D) characteristics of eccrine hydroadenocarcinoma. (A) Perianal resection specimen sections consisting of a whitish, homogeneous, compact tumour. (B) Microscopic image of lymph node metastasis due to hydroadenocarcinoma (see tumoural epithelial nests to the left and preserved rim of peripheral lymphoid tissue [arrow] to the right) (H&E× 400). (C) Infiltration, perianal cutaneous tumoural ulceration and lymphatic vascular invasion (double arrow) (H&E× 250) (C1), and microscopic image of the cribiform pattern of the neoplasm (H&E× 250) (C2). (D) Immunoexpression in eccrine gland lumina of carcinoembryonic antigen (CEA× 200) (D1); tumoural areas with clear-cell change (H&E× 200) (D2); intense, diffuse positivity for p53 (p53× 200) (D3); and a neoplastic component with positive immunostaining for cytokeratin 8 (CK8× 200) (D4).](https://static.elsevier.es/multimedia/24443824/0000004100000007/v2_201810040621/S244438241830107X/v2_201810040621/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
