Acute pancreatitis is one of most common causes of consultation due to abdominal pain in medical emergency units and it requires hospital admission. Although the majority of cases are mild and patients tend to recover quickly, a small percentage of cases is severe, with mortality in the region of 5–10%. This historical review considers how our understanding of this disease has changed since it was first described in 1579 thanks to the contributions of renowned experts such as Nicolaes Tulp, Reginald Fitz, Nicholas Senn and many others who, through their expertise and dedication, have improved the survival of patients with this disease.
La pancreatitis aguda es una de las principales causas de consulta por dolor abdominal en las unidades de urgencias médicas y requiere hospitalización. Aunque la mayoría de ellas son leves y se recuperan rápidamente, hay un bajo porcentaje que tienen una evolución grave y su mortalidad es del 5–10%. En este artículo se realiza una revisión histórica de las vicisitudes que ha sufrido esta enfermedad desde su primera descripción en 1579 y que va ligada a nombres tan prestigiosos como Nicholaes Tulp, Reginald Fitz, Nicholas Senn y otros muchos que, con sus conocimientos y esfuerzo, han mejorado la supervivencia de los pacientes con esta patología.
Alexander the Great died in 323 BC, a few days before his 33rd birthday. He had returned to Babylon after his latest conquests near the Indus River in the East. To celebrate this, he and his generals held a great banquet where copious amounts of food and alcohol were consumed. The next day, Alexander complained of abdominal pain, which gradually got worse, and he died 12 days later.
For many years, the most widely accepted theory to explain this outcome was poisoning. However, according to the historian Robin Lane Fox, the most common poisons at that time were strychnine and hellebore and both have sudden effects. He therefore thinks it is very unlikely that Alexander could have survived 12 days if he had been poisoned.1 It has also been suggested that he may have died from malaria, as there was an outbreak in the city of Babylon at the time. However, in 1986, Simmy Bank (Moorreesburg, South Africa, 1931) suggested that this may have been the first case of acute alcohol-related pancreatitis, although this can never be proven.2
Despite the fact that different anomalies in the pancreas had been described since ancient times using the Greek word skírros or the Latin word scirros (tumour, hard), no specific disease linked to this organ had been described in detail. However, in 1788, Thomas Cawley published his post-mortem findings for a 34-year old diabetic patient whose pancreas was full of calculi.3 As a result of his observations, he suggested a probable connection between diabetes and the appearance of the pancreas, although he was unable to establish the cause or consequence.
These two publications may show the two extreme forms of pancreatitis: the first, acute and rapidly fatal, and the second, chronic with long-term progression.
Various forms of this inflammatory pancreatic disease have been described over the last 125 years, resulting in different classifications. The first widely accepted classification was that of the 1963 Marseilles Symposium,4 after which a number of different categorisations have been proposed. This article presents the different authors who have described or played a part in describing and improving understanding of this disease, which affects a large part of the population.
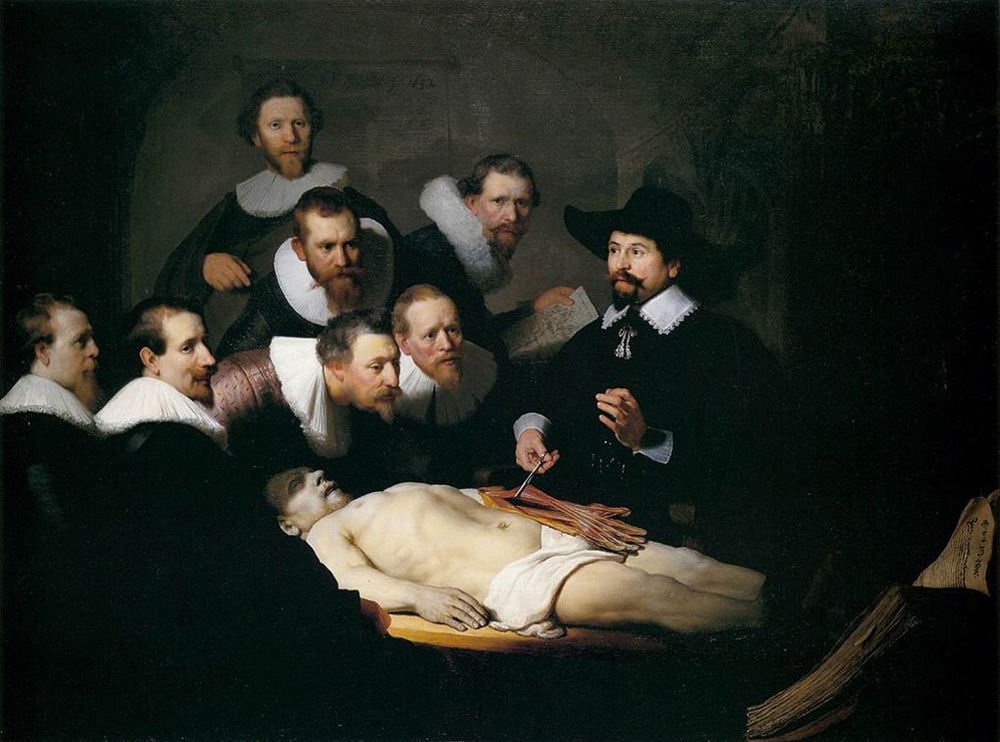
Initial descriptions and subsequent findingsThe first ever description of signs and appearance of the pancreas attributable to acute pancreatitis was published in Basel in 1579 by Iacobo Auberto Vindone (1500?–1587), a Huguenot doctor who had performed a post-mortem on an alcoholic patient with a necrotic pancreas (Fig. 1). Almost three-quarters of a century later, in 1652, Nicolaes Pietersz Tulp (Amsterdam, 1593-The Hague, The Netherlands, 1674), a renowned Dutch doctor and anatomist, published a description of the signs and anatomical findings of a pancreatic abscess that he had observed in a young man who died after suffering from back pain, low-grade fever, insomnia and agitation in his fourth book, Observationes medicae.5 Years earlier, in 1632, Nicolaes Tulp had been immortalised by Rembrandt (1606?–1669) in the painting The Anatomy Lesson of Dr. Nicolaes Tulp when he was a Professor of Anatomy of the Amsterdam Surgeons’ Guild, a position he held from 1628 to 1653. In this painting, we can see Tulp surrounded by a group of individuals to whom he is describing the anatomy of the arm on the corpse of the executed Adriaen Adriaenszoon, a regular criminal in Amsterdam, nicknamed Aris’t Kind (Aris’t the Kid)6 (Fig. 2).
A decade after Tulp's description, Guy Patin (1601–1672), who had been Dean of the Faculty of Medicine in Paris, published a similar observation. In 1679, Théophile Bonet (Geneva, Switzerland, 1620–1689) described the case of a “corrupted and purulent” pancreas in his book Sepulchretum sive Anatomica Practica ex Cadaveribus Morbo denatis (the cemetery or anatomy studies on bodies killed by disease), containing a compilation of almost 3000 post-mortems including their medical histories. This is considered the first complete book of Anatomical Pathology. Two years later, J. G. Greiselius of Silesia published a new description of pancreatic necrosis in his book De repentina suave morte ex pancreate sphacelato.7 Years later in 1761, Giovanni Battista Morgagni (Forli, Italy, 1682–1771) published De sedibus et causis morborum per anatomen indagatis, which contained more than 700 medical histories with their respective post-mortems. This book included the case of a patient with severe pain in the upper abdomen, vomiting and collapse; during the post-mortem he observed an enlarged pancreas that was full of unequal nodules and the consistency of cartilage.8 This book was translated into several languages, and has been used as a reference for Anatomical Pathology studies.
In 1788, the aforementioned Thomas Cawley described multiple stones in the pancreatic parenchyma of a diabetic patient, which could be the first description of long-term chronic pancreatitis in diabetes.3 However, it must be remembered that some authors, such as Bonet (1679) and Morgagni (1761), had already described the existence of pancreatic calculi at the time of post-mortem. In 1793, Matthew Baillie (1761–1823), a Scottish physician and pathologist, edited the book The morbid anatomy of some of the most important parts of the human body, in which he described a hard pancreas with distinct lobules and with concretions in the ducts and areas of fibrosis, which may have been chronic pancreatitis.9 From here onwards, two different anatomical aspects of pancreatic involvement were described, interpreted as acute pancreatitis and chronic pancreatitis, which resulted, after about 170 years, in a classification to define these different types of pancreatitis.4 This first classification would subsequently undergo several modifications.
In 1803, Antoine Portal (Gaillac, 1742–Paris, France, 1832), who was founding president of the Académie Nationale de Médecine in France, doctor to Louis XVIII and Charles X, and the first person to link gastrointestinal haemorrhage to oesophageal varices, described gangrenous pancreatitis that may have been related to gallstones.10 A similar description was published by Auguste-Nicolas Gendin (1796–1890) in 1826. These authors also observed an analogy between inflammation of the pancreas and the salivary glands, a fact that was also observed by other authors such as Thédoric Lerminier (1770–1836) and Salomon Neumann (1819–1908). However, it was only in 1899 that H. F. Harris of Boston was convincingly able to link mumps and pancreatitis.11
At that time, there were many theories about possible causes of the disease, including the effect of mercury, used in the treatment of syphilis, chronic liver disease, migration of a worm from the duodenum to the pancreatic duct, peptic ulcer penetrating the pancreas, and compression of the bile duct that caused pancreatitis associated with jaundice. However, the finding made by G. Fleischman in 1815, and described in his book Leichenöffnergen (Post-mortems),12 must be studied. It is the case of a young male alcoholic who had died after repeated episodes of abdominal pain, nausea and vomiting. During the post-mortem, he detected a very hard pancreas that he interpreted as being the result of several bouts of pancreatitis and suggested that it showed chronification of the disease. Based on this description, Heinrich Claessen in 1842 and Nikolaus Friedreich (1825–1882) in 1878 speculated that alcohol plays a role in the development of pancreatic inflammation.13,14 They were the first to highlight what would later be considered one of the main causes of pancreatitis. Nikolaus Friedreich coined the term “drunkard's pancreas”, a term subsequently popularised by Reginald Fitz in 1889.
Karl von Rokitansky (Hradec Králové, Bohemia, 1804–Vienna, Austria, 1878), professor of Pathology and first Dean-Elect of the University of Vienna, was the first person to recognise acute haemorrhagic pancreatitis as an entity in 1842.15 Years later, in 1870, Theodor Albrecht Edwin Klebs (Königberg, Prussia, 1834–Bern, Switzerland, 1913), a pathologist and bacteriologist, proposed a connection between haemorrhagic pancreatitis and purulent peripancreatitis with partial sequestration of the gland.16 Sixteen years later, Nicholas Senn (Sevelen, Sweden, 1844–1908), who was working as a surgeon at Rush Medical College in Chicago, agreed with the opinion of the two previous individuals that pancreatic infection and abscess were a consequence of acute pancreatitis. He defended his hypothesis at a meeting of the American Surgical Association on the basis of experiments and research performed for his article The surgery of the pancreas.17 He also concluded that the affected part of the pancreas was reabsorbed and replaced by connective tissue, while the rest of the gland continued to function normally. He also observed that if the pancreatic duct was affected by the inflammatory process, this resulted in ductal obstruction from scarring with subsequent deterioration of the distal portion of the gland.18 Senn also performed multiple experimental and clinical studies related to pancreatic surgery, which led to him being recognised as the “father of experimental pancreatology”.
In 1896, Hans Chiari (Vienna, Austria, 1851–Strasbourg, France, 1916), a former student of Rokitansky, proposed a theory of trypsin autodigestion as a possible pathogenetic mechanism of pancreatic necrosis initiated by activation of bile or enterokinase, as suggested by Claude Bernard and Nicholas Shepowalnikov (a former student of Pavlov) seven years earlier.19 It seems that this was the first reasonable theory put forward to explain the pathogenesis of pancreatitis. Based on this same theory, in 1939, Gerhardt Katsch (Berlin, Germany, 1887–1961) described the phenomenon of “enzymatic derailment” through which activated pancreatic enzymes circulating in the bloodstream cause damage to the lung, kidneys and capillaries (Fig. 3). Other authors, between this date and 1970, gave similar explanations for hypocalcaemia, fat necrosis, increased capillary permeability, increased pulmonary surfactant damage, etc.
Gerhardt Katsch (Berlin, Germany, 1887–1961). German doctor specialising in internal medicine who in 1925 suggested determining serum amylase and lipase concentrations to diagnose pancreatitis, and in 1939 formulated the theory of “enzymatic derailment” to explain the pathogenesis of this disease.
In 1901, Eugene L. Opie (Stauton, Virginia [USA], 1873–1971), a pathologist at Johns Hopkins Hospital in Baltimore, proposed the “common channel theory” based on his post-mortem findings in patients with acute haemorrhagic pancreatitis. In one case, he observed a small stone that dilated the common bile duct but not the pancreatic duct, which led him to suggest that reflux of bile into the pancreatic duct caused the pancreatitis. This theory was substantiated by performing experiments that involved injecting bile into the pancreas of animals and causing pancreatitis.20 This led him to postulate that obstruction of the pancreatic duct by stones could cause the disease. However, it is known that only a fraction of patients with acute pancreatitis have stones obstructing the bile duct. This is partly due to other causes of the disease (including alcohol) and the fact that stones often only persist for a short period of time, obstructing the bile and pancreatic ducts, as pointed out by Juan Acosta and Carlos Ledesma (Rosario, Argentina) in 1974 on observing stones in the stools and not in the bile duct in 94% of patients who had suffered from acute pancreatitis, indicating that stones are passed into the gut after the initial period of the disease.21
A few years earlier, in 1889, Reginald Herber Fitz (Chelsea, Massachusetts [USA], 1843–1913), emeritus professor of Pathological Anatomy at Harvard University and a former student of Rokitansky and Virchov, published an article in the Boston Medical and Surgical Journal22 (founded in 1828 and renamed the New England Journal of Medicine 100 years later) in which he documented the signs and symptoms of acute pancreatitis and established the different stages of the process, describing gangrenous, haemorrhagic and suppurative changes. He proposed different aetiologies, such as gallstones, alcohol, perforated peptic ulcer and abdominal trauma. He also described abscess, splenic vein thrombosis and a pseudocyst of the pancreas as complications associated with acute pancreatitis.
In 1908, Julius Wohlgemuth (Berlin, Germany, 1874–1948), a German physiologist, discovered a method to measure serum amylase concentration, which allowed diagnosis of acute pancreatitis prior to laparotomy or post-mortem, the only possible ways to confirm diagnosis at that time, representing a major breakthrough in the clinical care of patients. In 1911, Peter Rosenfeld Rona (Budapest, Hungary, 1871–1945), a physiologist of Jewish origin, and Leonor Michaelis (Berlin, Germany, 1875–1949), a biochemist and doctor who emigrated to Japan in 1922 (University of Nagoya) and to the United States in 1926 to work at Johns Hopkins University in Baltimore and later at the Rockefeller Institute in New York, determined the activity of lipase in blood. Using the data provided by Wohlgemuth, Rona and Michaelis, Gerhardt Katsch suggested determining amylase and lipase concentrations for the biochemical diagnosis of pancreatic diseases in 1925, and in 1929 Robert Elman, a surgeon from Saint Louis, established the connection between increased serum levels of pancreatic enzymes and the existence of inflammation of this gland, which definitively demonstrated the diagnostic usefulness of these findings.23
In 1916 and 1920, respectively, gynaecologist Thomas Stephen Cullen (Bridgewater, Ontario [Canada], 1868–1953) and surgeon George Grey Turner (North Shields, United Kingdom, 1877–1951) described, respectively, periumbilical discoloration and ecchymosis of the abdominal flanks associated with haemorrhagic pancreatitis.24
The first imaging diagnosis of pancreatic disease was performed in 1927 when calcifications in the pancreatic area were observed on a plain abdominal X-ray. This was possible thanks to the discovery made by Wilhelm Conrad Rontgen (Lennep, Prussia [now Germany], 1845-Munich, Germany, 1923) in 1895, which earned him the Nobel Prize in Physics in 1901.25 However, ante-mortem and/or pre-operative morphological diagnosis of pancreatic disease could not be performed systematically and convincingly until different imaging techniques were introduced (ultrasound [1971], computed tomography [1974], magnetic resonance [∼1975]) and endoscopy (endoscopic retrograde cholangiopancreatography [1968] and endoscopic ultrasound [1980]).26
Classification of pancreatitisAfter the clinical and anatomical description by Reginald Fitz in 1889, several attempts were made to classify pancreatitis based on observations made by laparotomy or post-mortem, but none of them were successful enough to last, even for a limited period of time.
In 1946, Mandred W. Comfort (Hillsboro, Texas [USA], 1894–1957), a doctor at Mayo Clinic in Rochester, Minnesota, established a new clinical and anatomopathological concept by introducing the term “chronic relapsing pancreatitis”.27 Two years later, surgeons Henry Doubilet and John H. Mulholland, at the Bellevue Hospital in New York, introduced the idea of “recurrent acute pancreatitis”, describing its aetiology and surgical treatment based on 21 case reports.28 Years later, in 1958, surgeons Joseph L. Owens and John M. Howard, from Atlanta, made a clear distinction between lithiasic and alcoholic pancreatitis and described calcifications in chronic alcoholic pancreatitis.29 But it was not until 1963, at a meeting held in Marseilles under the watchful eye of the chair Henri Sarles (1922–2017), a renowned gastroenterologist at Hôpital Sainte Marguerite in Marseilles, where a consensus was reached on the first classification based primarily on clinical and morphological criteria. This classification distinguishes between acute, acute relapsing, chronic and chronic relapsing pancreatitis.4 Despite the simplicity of this clinical classification and its wide acceptance, the terms acute relapsing pancreatitis and chronic relapsing pancreatitis caused confusion since, even today, it is difficult to differentiate between a relapse of acute pancreatitis and an exacerbation of chronic pancreatitis.
Twenty years later, thanks to the introduction of imaging techniques (ultrasound and computed tomography) and endoscopy (endoscopic retrograde cholangiopancreatography), a new classification was drafted based on morphological features and the natural history of the disease. An initial meeting took place in the city of Cambridge in 1983 under the auspices of the Pancreatic Society of Great Britain and Ireland.30 A year later, a second symposium was held, again in the city of Marseilles. The distinction between acute and chronic pancreatitis was maintained at both meetings.31 It was agreed that the acute form was characterised by abdominal pain accompanied by elevated blood and urine levels of enzymes. It was recognised that there may be systemic responses of varying severity, and that attacks could recur. Both meetings agreed that complications included necrosis, haemorrhage and pseudocyst, but the Cambridge meeting added phlegmon and abscess. In Marseilles, further definitions and essential morphological criteria for the diagnosis of acute pancreatitis were outlined. Therefore, a severe form with peripancreatic and intrapancreatic fat necrosis, parenchymal necrosis and haemorrhage, and a less severe form with only peripancreatic fat necrosis and interstitial oedema were described. At the same time, they established that these lesions could be localised or diffuse, and that the morphological and functional abnormalities returned to normal after each attack. Chronic pancreatitis is characterised by irreversible histological changes that can be progressive and lead to a loss of exocrine and endocrine functions, and that are very often associated with abdominal pain. A special form was defined, chronic obstructive pancreatitis, which is characterised by possible improvement in exocrine function once decompression of the obstructed pancreatic duct has been achieved.
In 1992, a group of experts met in Atlanta with the aim of designing a new classification for pancreatitis.32 Different criteria relating to acute pancreatitis were combined to define a severe form (necrohaemorrhagic) associated with multiple-organ failure and/or local complications, such as necrosis, pseudocyst or abscess, and a mild form (oedematous or interstitial) with minimal organ dysfunction and uneventful recovery. Concepts such as fluid collection, necrosis, pseudocyst and abscess were also defined. However, this classification did not correctly stratify the different degrees of severity and did not clearly define the morphological definition of local complications.
Therefore, taking into account that new physiopathological knowledge about acute pancreatitis indicates that its severity is marked by the systemic repercussions it causes (organ failure), especially if it is persistent, and also by the local complications that may develop (fluid collection or necrosis), especially if they become infected, 2 new classifications were proposed: the Determinant-based classification (PANCREA)33 in 2012 and the revised Atlanta Classification in 2013.34 The Determinant-based classification divides the severity of patients into 4 categories: mild (no necrosis or organ failure), moderate (sterile necrosis and/or transient organ failure), severe (infected necrosis or persistent organ failure) and critical (infected necrosis and persistent organ failure), and the revised Atlanta Classification divided severity into 3 categories: mild (no local or systemic complications and no organ failure), moderately severe (local or systemic complications and/or organ failure) and severe (persistent organ failure [>48h]). The latter also introduced a new type of complication, which they called “walled-off necrosis”. It involves necrotic pancreatic or peripancreatic tissue surrounded by an inflammatory wall (enhanced on dynamic computed tomography images) and requires a minimum of 4 weeks for this wall to form.
Both sets of criteria have proved to be better than the old Atlanta classification of 1992, but it remains to be seen whether they are mutually exclusive or complementary, as some authors have pointed out.35 It seems that the 2013 revised Atlanta classification better reflects reality in daily clinical practice with patients.
The search for prognostic factors of severityDoctors who treat patients with acute pancreatitis have always been interested in finding tools to predict how the disease may progress, e.g. from a mild form with rapid patient recovery or whether the patient will suffer severe life-threatening complications. Therefore, in 1974, John H. Ranson (Bangalore, India, 1938–1995) published a set of criteria (known as the Ranson criteria) that have been used to predict the severity of acute alcoholic pancreatitis, although they were later modified slightly to evaluate biliary pancreatitis. This scoring system was based on the measurement of 11 factors: 5 measured at the time of admission and 6 at 48h. The presence of three or more of these factors predicts an increased risk of death or severe pancreatitis with a sensitivity of 60–80%.36
Four years later, a team of surgeons from Glasgow Royal Infirmary, led by Clement W. Imrie, an eminent Scottish surgeon who is an expert in pancreatic pathology,37 published another series of factors to help predict prognosis at the beginning of an episode of acute pancreatitis, whether caused by alcohol or gallstones. If the patient has three or more criteria, this indicates that there is a high risk of severe complications during the course of the disease. These criteria are known as the Glasgow Criteria, and were reviewed by the same Imrie-led team in 1984.38
In 1983, Le Gall et al. developed the Simplified Acute Physiology Score (SAPS) in France, which was later modified in 1993 and renamed SAPS II.39 Subsequently, the Acute Physiology and Chronic Health Evaluation (APACHE II) was introduced in 1989. These are general acute illness severity scoring systems for the assessment of patients who require admission to intensive care units. Both systems involve assessing 12 physiological variables, age and previous health status.40
In 1985, Emil Jacques Balthazar (Romania, 1933), who emigrated to the United States and became an emeritus professor of Radiology at the New York University School of Medicine, specialising in abdominal radiology, published the criteria that bear his name. The Balthazar score is used to assess prognosis in acute pancreatitis according to data obtained by computed tomography.41 Five years later, he published a new article which also graded the extent of pancreatic necrosis, thanks to dynamic tomography; this was called the CT Severity Index (CTSI).42
In 1987, Pauli Poulakkainen et al., from the University of Helsinki, described the value of serum C-reactive protein (CRP) determinations in the assessment of the severity of acute pancreatitis.43 This protein had been described in 1930 by William S. Tillet and Thomas Francis, of the Rockefeller Institute for Medical Research, in relation to pulmonary lesions caused by pneumococcus.44
In 1993, Ivor C. Funnell et al., from Cape Town (South Africa), pointed out the risk posed by obesity in patients with acute pancreatitis, proposing that the body mass index (BMI >30kg/m2) be included as a criterion for predicting severity.45 Their results have subsequently been proven in several studies as a risk factor for the onset of local and systemic complications, and also mortality.46
Koenraad J. Mortele, from Brigham and Women's Hospital in Boston, published an article in 2004 proposing a new radiological index to improve the CTSI by modifying imaging criteria and linking them to the Atlanta classification.47 Also in the same year, Marianna Arvanitakis, from the Erasmus University Hospital in Brussels, published what has been called the Magnetic Resonance Severity Index (MRSI) on observing that data provided by magnetic resonance imaging are comparable to those obtained by CT scans for identifying the severity of acute pancreatitis, but present fewer contraindications and also allow identification if there is disruption of the pancreatic duct, which can occur in the initial phases of the disease.48
In 2008, Bechien U. Wu, of the Harvard Medical School in Boston, published the so-called Bedside Index of Severity in Acute Pancreatitis (BISAP) in order to simplify assessment of the different severity criteria known at that time. The BISAP calculates, within the first 24h of admission, blood urea nitrogen >25mg/dl, impaired mental status, systemic inflammatory response syndrome (SIRS), age >60 years or pleural effusion, which increases the risk of mortality. This system is capable of predicting mortality in a similar way to APACHE II, but with much simpler calculations.49
One year later, Paul G. Lankish, of the Municipal Clinic of Lüneburg (Germany), published a study, after reviewing studies published up until then, in which he defined and evaluated a simple clinical algorithm for rapid identification of patients with a first attack of pancreatitis who do not require intensive care. It assessed abdominal pain with no guarding and/or rebound tenderness and normal haematocrit and serum creatinine levels. The study was conducted in more than 800 patients with a diagnostic accuracy of 98%. This system has been called the Harmless Acute Pancreatitis Score (HAPS).50
However, most authors currently agree that age (>35), BMI (>30kg/m2), organ failure and SIRS, CRP (>150mg/dl), blood urea nitrogen (>25mg/dl), haematocrit (>44%) and CTSI or MRSI scores (within the first 72–96h) are the best factors for indicating which patients require intensive care due to the risk of poor pancreatitis outcome.
The pursuit of effective treatmentHistorically, the debate regarding the benefit of medical or surgical treatment in acute pancreatitis dates back to the late 19th century, in the time of Reginald Fitz and Nicholas Senn, and has continued ever since. In 1886, Nicholas Senn considered that surgery during the early stages of the disease was ineffective and also risky.17 However, a few years later, Fitz considered surgery to be a satisfactory treatment in the initial stages.51 Most patients, at the time of these authors and at the beginning of the 20th century, were diagnosed during the post-mortem or during abdominal surgery, which only a few of them survived. In 1887, August Socin (Vevey, Switzerland, 1837–1899), a professor of surgery in Basel, drained a pancreatic cyst causing bowel obstruction in a 45-year-old woman who died after 24h. The cyst turned out to be a haematoma of the pancreatic head that most likely developed from acute pancreatitis, which is why it is considered one of the first surgical treatments in this disease.52
At the beginning of the last century, surgery was considered the best treatment. As a result, Arthur William Mayo-Robson (Filey, Yorkshire [Great Britain], 1853–1933), Johann von Mickulicz-Radecki (Czerniowce, Bukowina [today Ukraine], 1850–1905), and especially Berkeley Moynihan (Malta, 1865–1936) began to perform laparotomies with drainage of the lesser sac and placement of gauze to achieve good drainage and prevent wound closure over the drain (Fig. 4).53 Until the 1930s, surgery was the treatment of choice, despite the fact that its mortality rate was higher than 50%. Once it was possible to determine serum amylase concentrations, a diagnosis could be reached without needing to perform a laparotomy, which extended the use of conservative treatment, especially in non-severe forms of the disease, so surgery was rarely performed. This was reinforced by a publication by John R. Paxton and J. Howard Payne (Los Angeles, California) in 1948 in which, after reviewing 307 cases of pancreatitis, they highlighted poor survival rates after early surgery. Surgery was therefore considered unnecessary and detrimental.54 Since the 1960s, given the poor results of medical treatment, surgeons reconsidered their procedures and again indicated surgery in the initial stages of the disease. However, high mortality rates persisted so drug treatments continued to be tested; a series of prospective studies conducted in the 1980s showed that conservative treatment in patients with sterile necrosis could be better than surgery and that better outcomes were obtained if surgery was delayed, which changed the therapeutic strategy again. In cases of infected necrosis, it was suggested that surgery should be delayed and debridement, sequestrectomy, lavage (continuous or intermittent)55 and what was called ‘open-packing’56 should be performed. The approach could be abdominal or translumbar.55,57 Other authors proposed early actions in all patients with necrosis, whether infected or not.
In 1979, Stephen G. Gerzof, from the Boston Veterans Hospital, made an important breakthrough on discovering the value of abdominal abscess drainage under ultrasound or computed tomography, and in 1987 when implementing the use of ultrasound and CT-guided aspiration with subsequent culture of peripancreatic tissues and fluid collections, which allowed early diagnosis of the infection.58,59
At the turn of this century, Pamoukian and Gagner, from Mount Sinai Medical Center in New York, published an article on the advantages of laparoscopic necrosectomy,60 and a few years later, Seifert et al., from the Goethe University Hospital in Frankfurt, published an article on a multicentre study involving 93 endoscopic necrosectomies, although 4% of the patients ended up requiring open surgery.61
It was evident that one of the main problems experienced by these patients is infected pancreatic and peripancreatic tissue necrosis, which is one of the main causes leading to multiple organ failure. For this reason, in 1975, R. Howes proposed using prophylactic treatment with antibiotics,62 which has been debated to this day and has been the subject of multiple trials. Finally, it has been considered that this antibiotic prophylaxis shows no advantages in terms of disease progression.63 In 1984, based on the findings of E. Kivilaakso et al.,64 who identified bacterial translocation from the gut as being responsible for infection of pancreatic necrosis, Stoutenbeek's team demonstrated the value of cefotaxime for decontamination of the digestive tract in these patients, managing to reduce infection to 16%.65
In 1986, Hans G. Beger (Meissen, Germany, 1936) carried out a prospective bacteriological analysis at the University Hospital of Ulm involving 114 patients with severe acute pancreatitis. During the analysis, he surgically obtained necrotic tissue and analysed germ colonies. He proved that if this occurred at an early stage, these patients had higher morbidity and mortality rates than those with sterile necrosis.66
Regarding conservative treatment, the use of rehydration and analgesia was contemplated from the beginning, as was nasogastric aspiration, which was subsequently used to a very limited degree in very specific situations.67 Various drugs were also tested to reduce the duration of the disease and minimise tissue damage. Some drugs acted as inhibitors of pancreatic secretion, such as cimetidine,68,69 atropine,70 glucagon,71 calcitonin,72 somatostatin and its analogue octreotide,73 and others had an inhibitory effect on pancreatic proteolytic enzymes, aprotinin,37 gabexate mesylate74,75 and phospholipase inhibitors.76 However, none of these achieved the desired results.
Another fundamental aspect in the treatment of severe acute pancreatitis is the need to maintain the patients’ nutritional status since they require long fasting cycles and suffer from a major inflammatory process and the risk of serious infections. Initially, enteral feeding through a jejunostomy tube was tested in the 1960s,77 but this technique posed a very high risk of local complications. Therefore, the use of total parenteral nutrition was subsequently considered. However, it was shown that this system causes atrophy of the intestinal mucosa and enhances bacterial and toxin translocation, in addition to the risk of infection of the tube.78–80 For this reason, enteral feeding through a nasojejunal tube was proposed in the 1990s as it maintains good function of the intestinal barrier while reducing systemic infections, multiple-organ failure, the need for surgery and mortality.81,82 It was later shown that feeding through a nasogastric tube had the same advantages as using a nasojejunal tube. However, in certain cases, such as if the patient does not tolerate enteral feeding or it is contraindicated due to bowel obstruction, paralytic ileus or intra-abdominal hypertension, the parenteral route is still used.
A very important and definitive factor in the treatment of patients with severe acute pancreatitis was the creation of intensive care units. The first unit of this type was organised at Johns Hopkins Hospital in Boston by the neurosurgeon Walter Edward Dandy (Missouri, USA, 1886–1946) in 1926.83 However, it was Peter Safar (Vienna, Austria, 1924–2003), who emigrated to the United States after staying in a Nazi concentration camp and who graduated as an anaesthetist from the University of Pennsylvania, who quickly became interested in cardiopulmonary resuscitation and in 1962 established the first ICU as we know them today at Johns Hopkins Hospital in Baltimore.84 In these units, the haemodynamic, renal and respiratory status of patients is constantly monitored, thus leading to early intervention in case of any systemic or local complications.
It is currently considered that severe acute pancreatitis should be treated by multidisciplinary teams consisting of critical care doctors, gastroenterologists, radiologists, endoscopy technicians and surgeons.
Conflict of interestThe author declares that he has no conflict of interest.
Please cite this article as: Navarro S. Revisión histórica de algunos conocimientos sobre pancreatitis aguda. Gastroenterol Hepatol. 2018;41:143.e1–143.e10.