The clinical impact of head-of-bed elevation in patients with gastro-oesophageal reflux disease is unclear, because of inconsistency and methodological limitations of previous studies.
Patients and methodsA randomised single-blind single-centre controlled clinical trial with a 2×2 cross-over design, in 39 pharmacologically treated patients with gastro-oesophageal reflux disease. Active intervention was to use a head-of-bed-elevation of 20cm for 6 weeks and then to sleep without inclination for 6 additional weeks, with a wash-out of 2 weeks between periods. The primary outcome was a change ≥10% in RDQ score and secondary outcomes were a change ≥10% in SF-36 score, patient preference and frequency of adverse events.
Results27 (69.2%) patients who used the intervention reached the primary outcome vs 13 (33.3%) patients in the control group (RR: 2.08; 95% CI: 1.19–3.61). No effect was found in SF-36 score (RR: 1.11; 95% CI: 0.47–2.60). Preference favouring the intervention was 77.1% and adverse event proportion was 54.0%.
ConclusionHead-of-bed elevation improved reflux symptoms but there was no effect on quality of life. The finding of a non-optimal risk-benefit ratio warrants additional studies before this intervention can be recommended (IBELGA, ClinicalTrials.gov identifier NCT02706938).
El impacto clínico de la elevación de la cabecera de la cama en pacientes con enfermedad por reflujo gastroesofágico es incierto, por inconsistencia y limitaciones metodológicas en estudios previos.
Pacientes y métodosEnsayo clínico controlado aleatorizado unicéntrico simple-ciego cruzado 2x2, en 39 pacientes con enfermedad por reflujo gastroesofágico tratados farmacológicamente. La intervención fue elevar la cabecera de la cama a 20cm de altura durante 6 semanas y luego a dormir sin inclinación otras 6 semanas, con un lavado de 2 semanas entre períodos. El desenlace primario fue el cambio ≥ 10% de la puntuación RDQ y los desenlaces secundarios fueron el cambio ≥ 10% de la puntuación SF-36, preferencia del paciente y frecuencia de eventos adversos.
Resultados27 (69,2%) pacientes que utilizaron la intervención cumplieron el desenlace primario, vs. 13 (33,3%) pacientes en el grupo control (RR: 2,08; IC95%: 1,19–3,61). No se encontró efecto en la puntuación SF-36 (RR: 1,11; IC95%: 0,47–2,60). La preferencia por la intervención fue del 77,1% y la proporción de eventos adversos fue del 54,0%.
ConclusiónLa elevación de la cabecera de la cama redujo los síntomas de reflujo, pero no tuvo efecto en la calidad de vida. Por un balance riesgo-beneficio no óptimo, se requieren estudios adicionales antes de recomendar esta intervención (IBELGA, identificador ClinicalTrials.gov NCT02706938).
Gastro-oesophageal reflux disease (GORD) occurs when the stomach contents return to the oesophagus and cause complications or symptoms of discomfort for the patient.1 GORD affects 13% of the world population.2 In primary care it represents 4% of medical consultations3 and in Latin America it has a prevalence of 12–31%.4 Over 70% of patients are affected by night symptoms, significantly impairing their quality of life.5 The sleep loss caused by heartburn during the night is associated with low productivity at work,6 even in patients treated with proton pump inhibitors.7 Due to a number of different physiological factors, such as a lack of conscious perception of symptoms, and salivating and swallowing less at night,8 the significantly longer exposure to acid during the night has been associated with complications such as oesophagitis,9,10 more severe extra-oesophageal symptoms11 and other disorders such as asthma.12
In patients with erosive GORD, the gold standard of treatment is acid suppression with proton pump inhibitors (PPI).13,14 Taken twice a day, PPI improve sleep quality in patients with night-time symptoms11 and produce better acid suppression.15 Night-time discomfort has prompted various alternative approaches, including baclofen, as an adjuvant for patients refractory to PPI.16 In combination with PPI, various non-pharmacological interventions have also traditionally been used for night-time symptoms, such as postural measures and lifestyle modifications, including losing weight, stopping smoking and abstaining from alcohol, caffeine and fatty and acidic foods, among others.17 However, the quality of evidence supporting such recommendations is low and there is debate about their applicability and efficacy.18,19 A post hoc analysis of an observational post-marketing study found that recommending lifestyle changes could improve patients’ quality of life if accompanied by prescription PPI.20 Among the postural measures, head-of-bed elevation (HBE) has been object of the most clinical trials. However, as a recommendation it has a low level of evidence,14,21–23 due to inconsistent results, methodological limitations and heterogeneity in outcome measures in the randomised studies.18,24 In view of the above and the high frequency with which this strategy is recommended, including in recent clinical practice guidelines25 and expert recommendations,17 we decided to carry out this study to determine the efficacy of HBE for the symptoms of patients with GORD and the impact of the intervention on patient quality of life.
Patients and methodsThis was a single-blind, two-way crossover, single-centre, randomised, controlled clinical trial, in a sample of patients with GORD attending the outpatient clinic or the endoscopy suite at the Gastroenterology and Gastrointestinal Endoscopy Unit at Clínica Fundadores in Bogotá, Colombia. Individuals were randomised into two arms, in which reverse treatment sequences were administered over two six-week periods, separated by a two-week wash-out period. The intervention was to elevate the head of the bed using standard 20cm wedges or to sleep without an incline, and all patients received standard pharmacological treatment throughout the study, at the discretion of the treating gastroenterologist. Each patient was provided with a pair of wooden risers to use one under each leg at the head of the bed, such that the head of the bed was raised at an angle of approximately 4.01°–4.51°, depending on the length of the bed. The protocol and informed consent form were approved by the Ethics and Research Committees of the institutions involved. All patients signed the informed consent form before the start of the intervention.
Inclusion criteriaWe included patients over age 18 who met all four of the following criteria: (1) diagnosed with GORD and erosive oesophagitis, defined according to the Montreal consensus (oesophageal erosions and typical symptoms such as heartburn and/or reflux at least twice/week); (2) heartburn for at least three months; (3) heartburn and/or reflux at least three nights/week; and (4) GORD-associated sleep disturbance (sleep-onset insomnia, sleep-maintenance insomnia, poor sleep quality) at least three nights/week for at least one month. All patients with any of the following criteria were excluded from the trial: GORD without oesophageal erosions; gastric or duodenal peptic ulcer; history of upper gastrointestinal surgery except cholecystectomy; breastfeeding or pregnant women; night workers (12pm to 6am); obstructive sleep apnoea-hypopnoea syndrome (with or without CPAP use); chronic obstructive pulmonary disease; restless leg syndrome; orthopnoea; need for nightly oxygen (due to cardiopulmonary comorbidity); recent initiation or change (<3 months) of sleep drugs, such as anxiolytics, antihistamines or benzodiazepines; consumption of more than three cups (585ml) of coffee per day; or those planning to travel more than three time zones during the study.
Patient recruitmentPatients were recruited from April 2016 to July 2017. Initially, an attempt was made to include patients from the gastroenterology outpatient clinic in real time. However, because of the low recruitment rate with that method, an active participant search system was implemented by telephone call, using a database of all upper gastrointestinal endoscopies performed at the Clínica Fundadores Gastroenterology Unit from 2014 to 2016. The patients were preselected based on the endoscopic finding of erosive oesophagitis of any degree on endoscopy, which is one of the inclusion criteria of this study. To minimise selection bias, two interviewers made calls in chronological consecutive order to all patients in the database without exception, and the same standardised phone call format was used in all cases.
RandomisationA list of 42 numbers was generated randomly with subsequent binomial transformation, using the statistical programme STATA SE 13.0 for Windows (StataCorp LP, Texas, USA) to assign the participants in a 1:1 ratio to each of the arms of the study. Each pair of wooden risers was marked with a consecutive number from 1 to 42, and they were stored until use, keeping the numbers out of the sight of the investigator in charge of patient recruitment. When recruiting the patient, if there was a pair of risers marked with the consecutive number assigned to the patient in order of arrival, they were given to the patient along with written and verbal instructions for their use during the first period. If there was not a pair of wooden risers marked with the patient's consecutive number, they were assigned to the other arm of the study for the first period of the clinical trial.
Allocation concealmentThe investigator responsible for applying the inclusion and exclusion criteria, providing the informed consent form and completing the basic patient data form did not know the random sequence until the aforementioned documents were completed and the patient was assigned to an arm of the study using the consecutive number. At that point, another team member checked whether or not there was a pair of risers marked with the same consecutive number assigned to the patient, and only then was the allocation concealment broken.
BlindingDue to its nature, the non-pharmacological intervention did not need double blinding. However, the researcher in charge of the statistical analysis and preparation of the results report worked with alphabetical codes that masked the intervention in each of the study periods.
InterventionsA total of 84 wooden risers measuring 20cm×18cm×18cm were made from nine seasoned pine 300cm×20cm×20cm beams at the Aserrío San Ignacio Ltda. plant in Soacha, Cundinamarca, Colombia. The stability of each wooden riser was checked by standing it on the ground and products which were unstable or whose heights were irregular from the industrial cutting and planing process were rejected.
Outcome measuresFor the primary outcome, effectiveness was measured by a change of ≥0.6 points in the Reflux Disease Questionnaire (RDQ)26 between baseline and six weeks after starting HBE. Among the secondary outcomes, quality of life was measured by a change of ≥10 points in the Short Form-36 (SF-36) questionnaire27 between baseline and six weeks after starting HBE. We used the Spanish versions of both the RDQ28 and the SF-36.29 Patient preference was measured as the proportion of patients who preferred HBE after the end of the 14-week study. The safety of the intervention was assessed by systematic questioning about any adverse events in the follow-up phone calls in both arms and study periods. Any event that led to the patient stopping use of HBE was defined as a serious adverse event.
Baseline assessment and follow-upThe patient's medical history, medication use and other baseline variables were recorded on a data collection instrument designed for research, and adherence to drug therapy was measured using the Morisky-Green-Levine test.30 The information about the symptoms was assessed on admission with the RDQ and quality of life was assessed with the SF-36 questionnaire. After randomisation to each arm of the study, telephone follow-up was performed according to an allocation-dependent scheme. The patients who received the HBE were followed weekly for two weeks and then every two weeks for a month, until the end of the six weeks for each period. The patients without HBE were followed every three weeks in each period. In order to verify adherence to HBE and the correct use of the wooden risers, during each phone call the patient was asked to take a photograph of the legs at the head of the bed and send it by instant messaging to the investigator's mobile phone number or email. The patients completed the self-administered RDQ and SF-36 questionnaire at the beginning of the study and then at the end of the first period, after which they were asked to return the wooden risers to be stored by the investigators during the two-week wash-out period. After the wash-out period, the RDQ and SF-36 questionnaire were administered again in order to measure the baseline conditions of the group that would go on to use the HBE. Next, a pair of numbered risers were given to each patient who had not received HBE during the first period and the same photographic and telephone monitoring protocol described for the first period was applied. At the end of the second period of the study, the self-administered RDQ and SF-36 questionnaire were completed again, but this time the patients were also asked about their preference between HBE and sleeping without any elevation. Changes in drug treatment were not followed in either arm of the study.
Calculation of sample sizeWe worked on a null hypothesis of equality of changes in the RDQ and SF-36 scores between groups and an alternative one-way hypothesis of superiority of the HBE. The sample size was estimated based on the assumption that the HBE would produce a difference of at least 10% in the RDQ and SF-36 questionnaire scores. An effect size (Cohen's d) of 0.49 was calculated taking into account the mean and standard deviation for the RDQ of 3.3±1.0 previously found in a Spanish population with symptomatic GORD28 and mean and standard deviation for the SF-36 of 56.9±20.3 reported in a Italian population medically treated with PPI.31 The choice of the minimal important difference was based on the assumption that any difference of less than 10% would not have clinical relevance. Based on these data, approximately 14 patients per group would provide a power greater than 80% to detect a minimal important difference greater than or equal to 0.6 points in the RDQ (range: 1–6) and 10 points in the SF-36 (range: 0–100) using Student's t-paired statistic, as described in the statistical analysis plan. As we planned to perform an additional analysis for this cross-over study using the McNemar marginal homogeneity test, the effect size was recalculated based on the results of a previous clinical trial,32 according to which 58.8% and 28.6% of patients treated with placebo in the pre-omeprazole era improved GORD symptoms with and without HBE respectively. Based on the above, and maintaining a statistical power of 80%, the required sample size was adjusted to a total of 34 patients. Finally, the calculated sample size was increased by 20% to prevent any loss to follow-up or dropouts from affecting the power of the study, such that the final sample size was 42 patients, 21 per group. The matrices for the sample calculation based on the aforementioned study are attached with the additional material, along with the result of the sample calculation with the G*Power 3.1.9.2 software (Universität Düsseldorf, Düsseldorf, Germany).33
Statistical analysisThe numerical and categorical variables collected in the basic data form, the RDQ and the SF-36 and patient preference questionnaires, were entered into a database in the Microsoft Excel 2007 programme (Microsoft Corp. USA) and the intervention groups were masked with an alphabetical code prepared by an independent collaborator who was not involved in the statistical analysis or the results report. For statistical analysis, the database was imported into the STATA SE 13.0 programme for Windows (StataCorp LP, Texas, USA) and the descriptive statistics of each variable were studied. We looked for statistically significant differences between categorical variables using the Chi square test or Fisher's exact test and for continuous numerical variables, we first applied the Shapiro–Wilk test for normality. For the continuous numerical variables with normal distribution, we used the paired Student's t-test to look for statistically significant differences. Alternatively, for variables that did not show normal distribution, we used the Mann–Whitney U test. For variables with non-normal distribution due to outliers, values greater than or less than the sum of the 50th percentile and 1.5 times the interquartile range were excluded. As an additional analysis for the primary outcome and the secondary outcome, quality of life, the difference in the scores of the RDQ and SF-36 questionnaire was transformed into a binomial variable according to whether or not they met the predetermined criteria for each outcome. We then applied the McNemar marginal homogeneity test to the transformed data and used the McNemar mid-p test if the sum of discordant pairs was less than 25.34 Differences with one-tailed p < 0.05 for all tests with one-way hypotheses were accepted as statistically significant. The subgroup analysis was exploratory and we looked for stratified differences according to age group, gender, race, BMI category, comorbidities, cups of coffee per day, drug adherence, grade of oesophagitis and duration of symptoms, among 16 other variables collected at the start of the study. We performed tests of interaction between subgroups with the Chi-squared test or Fisher's exact test, according to the number of individuals analysed, and expressed the magnitude of the effect by way of the univariate relative risk of a binomial family generalised linear model. As part of the internal quality control of the clinical trial, a pre-test was performed in order to demonstrate the presumption of a negligible carry-over effect, following the recommendations of expert evaluators of scientific publications.35 An analysis of variance (ANOVA) for cross-over studies was also performed which looked for significant sequence, period and treatment effects, assuming a negligible carry-over effect. Participants who violated the protocol through poor adherence to HBE were included in the modified intention-to-treat analysis, but it was not possible to include patients who withdrew from the study in this analysis, because the information concerning the primary outcome and the secondary outcome, quality of life was not collected.
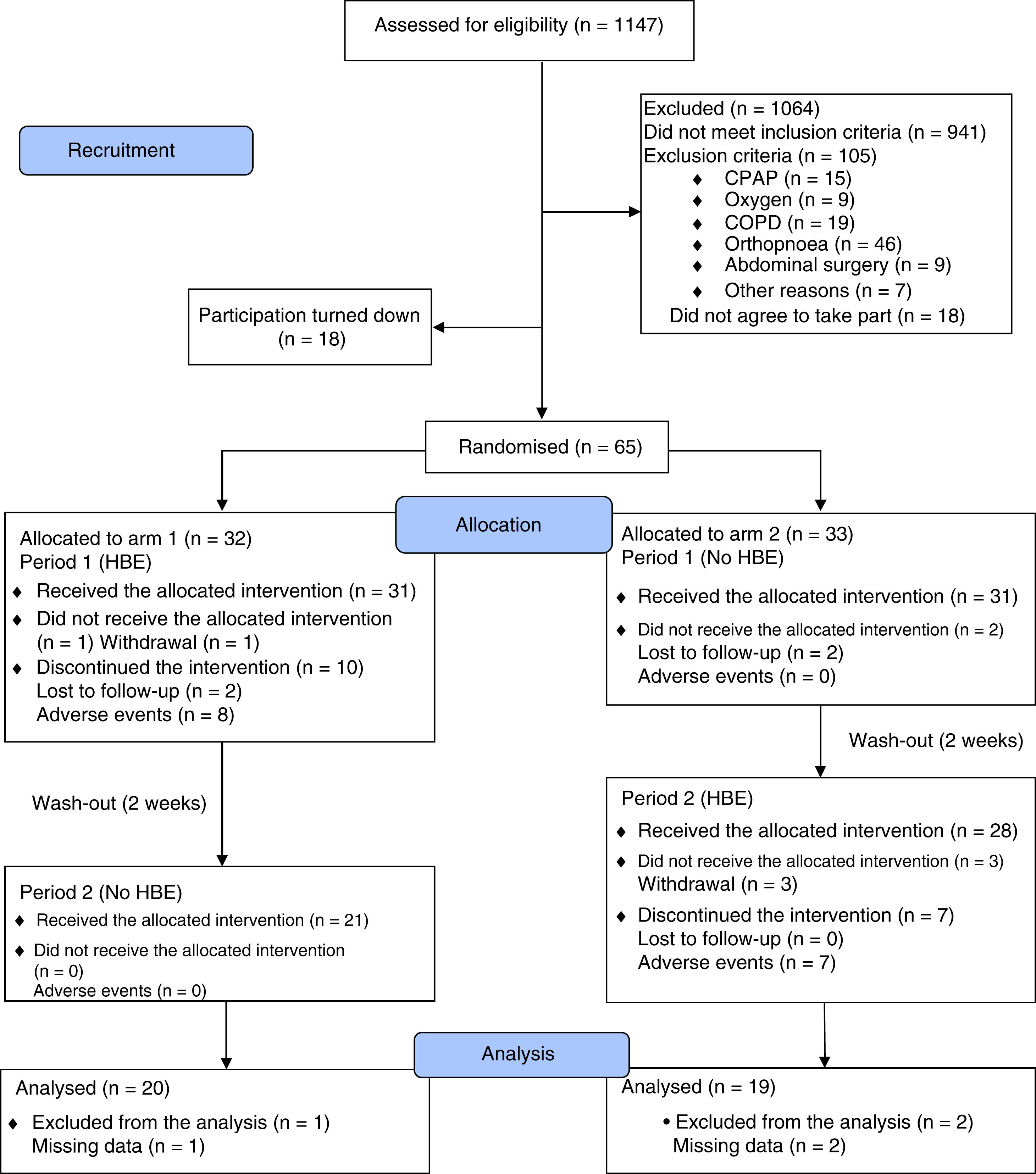
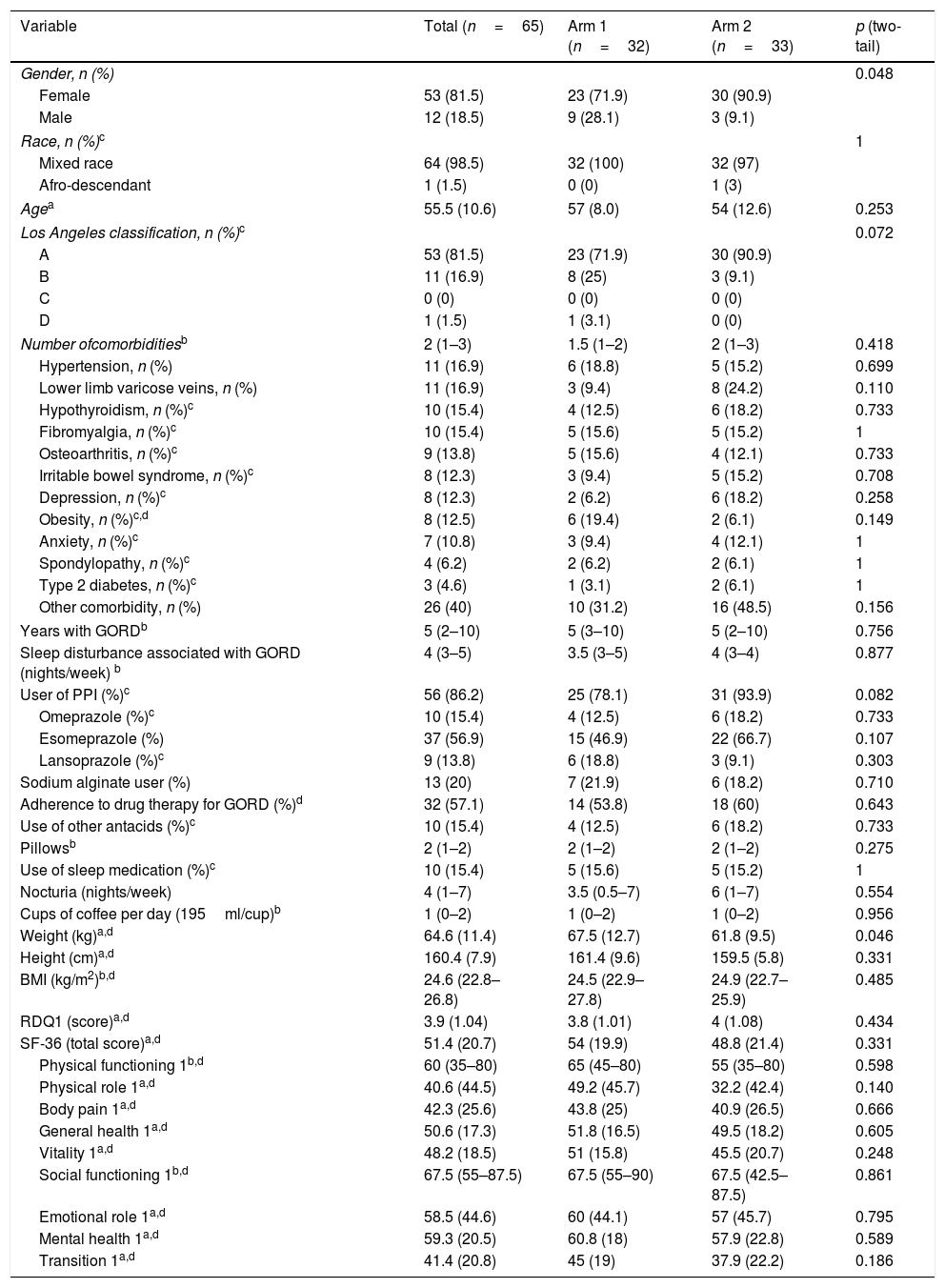
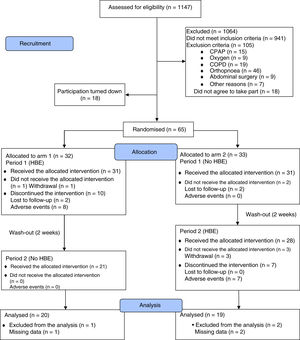
ResultsFrom April 2016 to July 2017, we assessed 1147 potentially eligible patients, 1064 of whom were excluded due to not meeting the inclusion criteria (n=941) or the presence of some exclusion criterion (n=105). Thirty-six patients decided not to take part before starting the clinical trial. Fig. 1 shows the flowchart for participants as recommended in the 2010 CONSORT statement.36 In the end 65 patients were randomised into two arms of 33 and 32 participants, and 39 patients (60%) completed the study. The baseline characteristics of the participants in both study arms were balanced, apart from a lower mean body weight in arm 2, which did not lead to differences in mean BMI between arms. An association was found between the variables gender and study arm, due to a higher proportion of females in each of the arms. During the clinical trial, 86.2% of the study patients received standard drug therapy with PPI and/or sodium alginate with an adherence rate of 57.1% (95% CI: 44.2–70.1; Z: 1.07; two-tailed p: 0.2850) (Table 1). The HBE adherence rate was 70.6% (95% CI: 58.1–83.1; Z: 2.94; two-tailed p: 0.0033).
Baseline characteristics of the recruited patients.
| Variable | Total (n=65) | Arm 1 (n=32) | Arm 2 (n=33) | p (two-tail) |
|---|---|---|---|---|
| Gender, n (%) | 0.048 | |||
| Female | 53 (81.5) | 23 (71.9) | 30 (90.9) | |
| Male | 12 (18.5) | 9 (28.1) | 3 (9.1) | |
| Race, n (%)c | 1 | |||
| Mixed race | 64 (98.5) | 32 (100) | 32 (97) | |
| Afro-descendant | 1 (1.5) | 0 (0) | 1 (3) | |
| Agea | 55.5 (10.6) | 57 (8.0) | 54 (12.6) | 0.253 |
| Los Angeles classification, n (%)c | 0.072 | |||
| A | 53 (81.5) | 23 (71.9) | 30 (90.9) | |
| B | 11 (16.9) | 8 (25) | 3 (9.1) | |
| C | 0 (0) | 0 (0) | 0 (0) | |
| D | 1 (1.5) | 1 (3.1) | 0 (0) | |
| Number ofcomorbiditiesb | 2 (1–3) | 1.5 (1–2) | 2 (1–3) | 0.418 |
| Hypertension, n (%) | 11 (16.9) | 6 (18.8) | 5 (15.2) | 0.699 |
| Lower limb varicose veins, n (%) | 11 (16.9) | 3 (9.4) | 8 (24.2) | 0.110 |
| Hypothyroidism, n (%)c | 10 (15.4) | 4 (12.5) | 6 (18.2) | 0.733 |
| Fibromyalgia, n (%)c | 10 (15.4) | 5 (15.6) | 5 (15.2) | 1 |
| Osteoarthritis, n (%)c | 9 (13.8) | 5 (15.6) | 4 (12.1) | 0.733 |
| Irritable bowel syndrome, n (%)c | 8 (12.3) | 3 (9.4) | 5 (15.2) | 0.708 |
| Depression, n (%)c | 8 (12.3) | 2 (6.2) | 6 (18.2) | 0.258 |
| Obesity, n (%)c,d | 8 (12.5) | 6 (19.4) | 2 (6.1) | 0.149 |
| Anxiety, n (%)c | 7 (10.8) | 3 (9.4) | 4 (12.1) | 1 |
| Spondylopathy, n (%)c | 4 (6.2) | 2 (6.2) | 2 (6.1) | 1 |
| Type 2 diabetes, n (%)c | 3 (4.6) | 1 (3.1) | 2 (6.1) | 1 |
| Other comorbidity, n (%) | 26 (40) | 10 (31.2) | 16 (48.5) | 0.156 |
| Years with GORDb | 5 (2–10) | 5 (3–10) | 5 (2–10) | 0.756 |
| Sleep disturbance associated with GORD (nights/week) b | 4 (3–5) | 3.5 (3–5) | 4 (3–4) | 0.877 |
| User of PPI (%)c | 56 (86.2) | 25 (78.1) | 31 (93.9) | 0.082 |
| Omeprazole (%)c | 10 (15.4) | 4 (12.5) | 6 (18.2) | 0.733 |
| Esomeprazole (%) | 37 (56.9) | 15 (46.9) | 22 (66.7) | 0.107 |
| Lansoprazole (%)c | 9 (13.8) | 6 (18.8) | 3 (9.1) | 0.303 |
| Sodium alginate user (%) | 13 (20) | 7 (21.9) | 6 (18.2) | 0.710 |
| Adherence to drug therapy for GORD (%)d | 32 (57.1) | 14 (53.8) | 18 (60) | 0.643 |
| Use of other antacids (%)c | 10 (15.4) | 4 (12.5) | 6 (18.2) | 0.733 |
| Pillowsb | 2 (1–2) | 2 (1–2) | 2 (1–2) | 0.275 |
| Use of sleep medication (%)c | 10 (15.4) | 5 (15.6) | 5 (15.2) | 1 |
| Nocturia (nights/week) | 4 (1–7) | 3.5 (0.5–7) | 6 (1–7) | 0.554 |
| Cups of coffee per day (195ml/cup)b | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.956 |
| Weight (kg)a,d | 64.6 (11.4) | 67.5 (12.7) | 61.8 (9.5) | 0.046 |
| Height (cm)a,d | 160.4 (7.9) | 161.4 (9.6) | 159.5 (5.8) | 0.331 |
| BMI (kg/m2)b,d | 24.6 (22.8–26.8) | 24.5 (22.9–27.8) | 24.9 (22.7–25.9) | 0.485 |
| RDQ1 (score)a,d | 3.9 (1.04) | 3.8 (1.01) | 4 (1.08) | 0.434 |
| SF-36 (total score)a,d | 51.4 (20.7) | 54 (19.9) | 48.8 (21.4) | 0.331 |
| Physical functioning 1b,d | 60 (35–80) | 65 (45–80) | 55 (35–80) | 0.598 |
| Physical role 1a,d | 40.6 (44.5) | 49.2 (45.7) | 32.2 (42.4) | 0.140 |
| Body pain 1a,d | 42.3 (25.6) | 43.8 (25) | 40.9 (26.5) | 0.666 |
| General health 1a,d | 50.6 (17.3) | 51.8 (16.5) | 49.5 (18.2) | 0.605 |
| Vitality 1a,d | 48.2 (18.5) | 51 (15.8) | 45.5 (20.7) | 0.248 |
| Social functioning 1b,d | 67.5 (55–87.5) | 67.5 (55–90) | 67.5 (42.5–87.5) | 0.861 |
| Emotional role 1a,d | 58.5 (44.6) | 60 (44.1) | 57 (45.7) | 0.795 |
| Mental health 1a,d | 59.3 (20.5) | 60.8 (18) | 57.9 (22.8) | 0.589 |
| Transition 1a,d | 41.4 (20.8) | 45 (19) | 37.9 (22.2) | 0.186 |
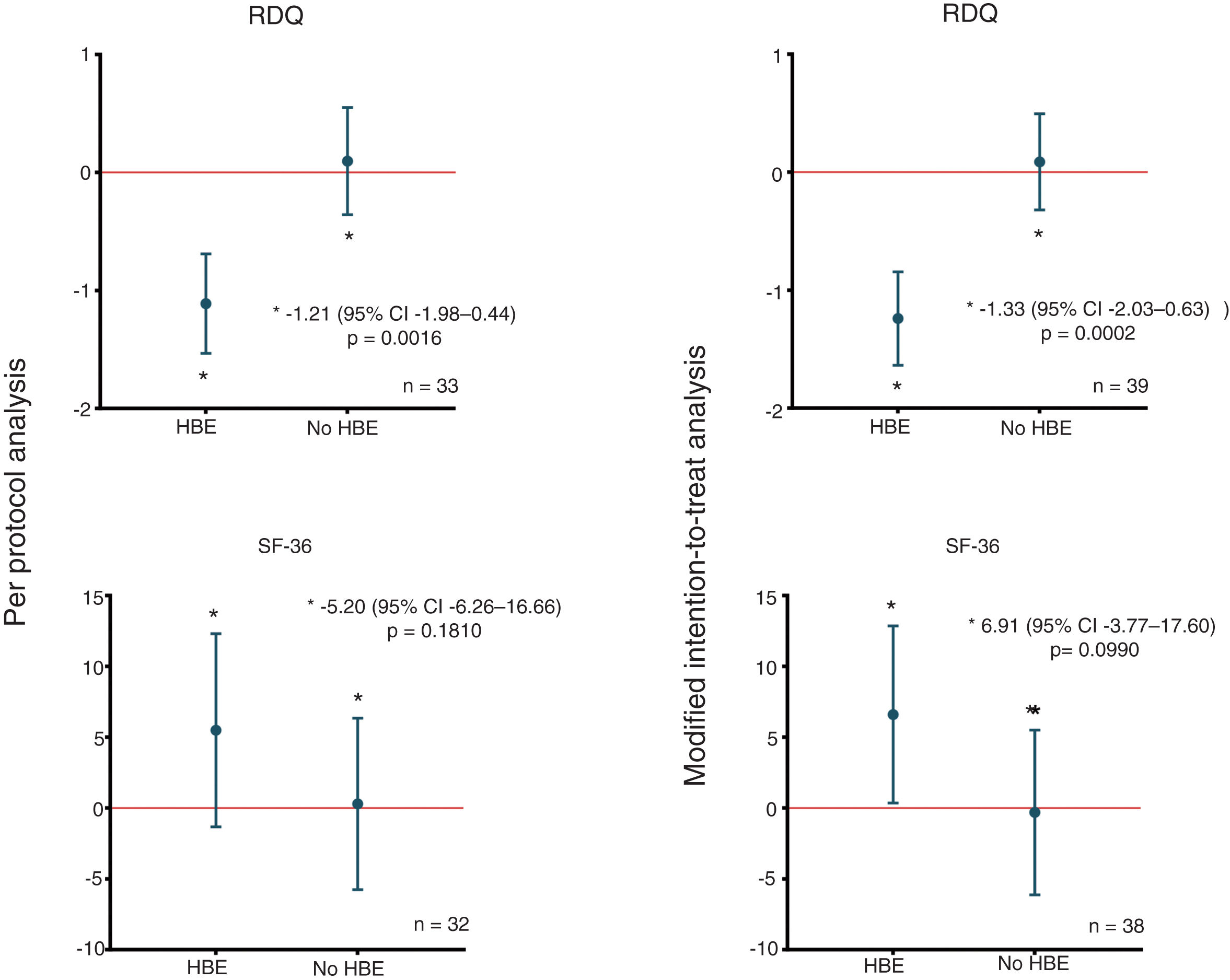
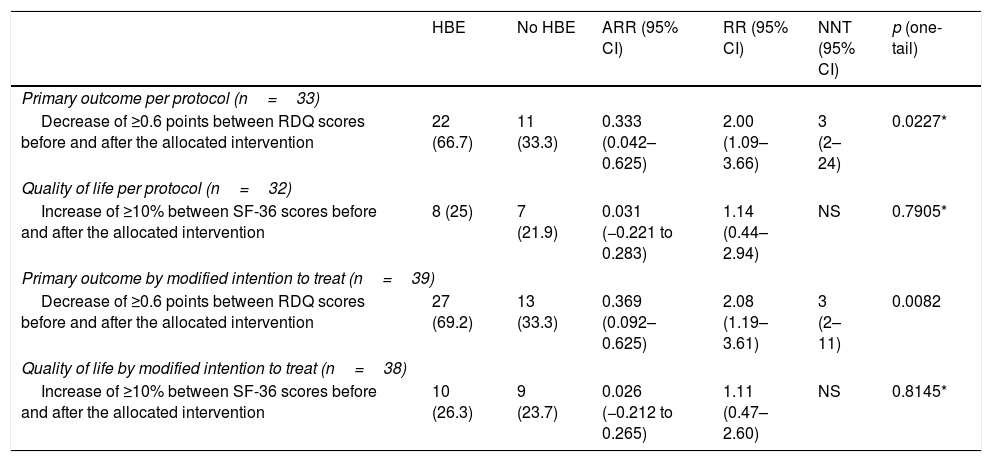
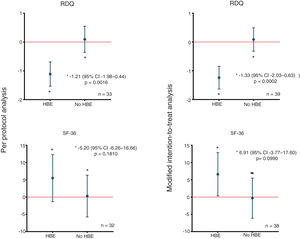
In the modified intention-to-treat analysis, when applying the McNemar marginal homogeneity test, a decrease greater than or equal to 10% was observed in the RDQ score associated with the use of HBE in 27 (69.2%) of the 39 patients who used HBE. As shown in Table 2, that finding was consistent with the per protocol analysis. The mean decrease in the RDQ score was −1.24 (95% CI: −1.64 to −0.84) when patients used HBE and 0.09 (95% CI: −0.32 to 0.49) when they did not. Once again, in the per protocol analysis, similar score changes of −1.11 (95% CI: −1.53 to −0.69) and 0.10 (95% CI: −0.36 to 0.55) were obtained for HBE and non-HBE respectively (Fig. 2). No significant changes were found in the RDQ sub-scores for acid reflux (−0.3, 95% CI: −1.38 to 0.78) or heartburn (0.3, 95% CI: −0.99 to 1.59) associated with use of HBE. In line with the preliminary test described by Wellek et al.35 no evidence was found of a relevant carry-over effect on the primary outcome (t=−1.6735; df=37; p=0.1027). In order to test the possibility of sequence (arm) or period effects in the primary outcome, an analysis of variance was performed for crossover studies assuming a negligible carry-over effect, which only yielded significant results for the effect of the HBE on the primary outcome (Appendix B, Supplementary Table 2 in the Appendix).
Primary outcome and quality of life.
| HBE | No HBE | ARR (95% CI) | RR (95% CI) | NNT (95% CI) | p (one-tail) | |
|---|---|---|---|---|---|---|
| Primary outcome per protocol (n=33) | ||||||
| Decrease of ≥0.6 points between RDQ scores before and after the allocated intervention | 22 (66.7) | 11 (33.3) | 0.333 (0.042–0.625) | 2.00 (1.09–3.66) | 3 (2–24) | 0.0227* |
| Quality of life per protocol (n=32) | ||||||
| Increase of ≥10% between SF-36 scores before and after the allocated intervention | 8 (25) | 7 (21.9) | 0.031 (−0.221 to 0.283) | 1.14 (0.44–2.94) | NS | 0.7905* |
| Primary outcome by modified intention to treat (n=39) | ||||||
| Decrease of ≥0.6 points between RDQ scores before and after the allocated intervention | 27 (69.2) | 13 (33.3) | 0.369 (0.092–0.625) | 2.08 (1.19–3.61) | 3 (2–11) | 0.0082 |
| Quality of life by modified intention to treat (n=38) | ||||||
| Increase of ≥10% between SF-36 scores before and after the allocated intervention | 10 (26.3) | 9 (23.7) | 0.026 (−0.212 to 0.265) | 1.11 (0.47–2.60) | NS | 0.8145* |
The table shows the magnitude of the effect of HBE on the primary outcome and quality of life, according to the type of study analysis.
HBE: head-of-bed elevation; n: number of individuals; NNT: number needed to treat; ARR: absolute risk reduction; RR: relative risk.
Differences in mean changes in RDQ and SF-36 scores according to allocation. The figure shows the differences in the mean changes in scores between groups and according to the type of study analysis, for the RDQ (above) and SF-36 (below). HBE: head-of-bed elevation; n: number of patients; RDQ: Reflux Disease Questionnaire; SF-36: Short Form (36) Health Survey.
Using the McNemar test, no changes greater than or equal to 10% were found in the SF-36 score associated with the use of HBE (Table 2). In the modified intention-to-treat analysis, the mean increase in SF-36 score was 6.60 (95% CI: 0.34–12.85) when patients used HBE and −0.31 (95% CI: −6.12 to 5.50) when they did not. Similarly, comparable score changes were found in the per protocol analysis: 5.48 (95% CI: −1.34 to 12.30) for HBE and 0.29 (95% CI: −5.77 to 6.34) for no HBE (Fig. 2). As with the primary outcome, there was no evidence of a relevant carry-over effect on the quality of life outcome (Z=0.409; p=0.6823). Lastly, the ANOVA for crossover studies showed no significant effects of sequence, period or HBE on the change in SF-36 score (Appendix B, Supplementary Table 2 in the Appendix).
Participant preference was found in favour of HBE in the per protocol analysis (proportion: 77.1%; Z: 3.21; 95% CI: 63.2–91.1; one-tailed p=0.0007) and maintained its significance in the modified intention-to-treat analysis (proportion: 63.2%; Z: 1.99; 95% CI: 50.6–75.7; one-tailed p=0.0235).
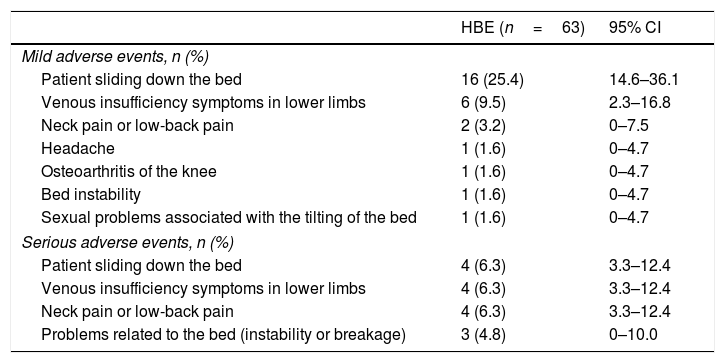
SafetyThe population at risk of adverse events was defined as the population present at the start of each period of active intervention with HBE. Thirty-four of these 63 participants reported some adverse effect during the use of HBE (proportion: 54.0%; 95% CI: 41.7–66.3) and 15 of these 34 patients had to discontinue HBE due to a related adverse effect (proportion: 23.8%; 95% CI: 15.0–35.6). However, none of the participants had to seek medical help to resolve the adverse effect (Table 3). Only one patient experienced an adverse event unrelated to HBE, requiring hospitalisation, due to Stevens-Johnson syndrome associated with drug therapy for one of their comorbidities. The mean length of time using HBE in the group of patients who discontinued the intervention due to adverse effects was 6±3 nights (mean±SD).
Adverse events related to the use of HBE.
| HBE (n=63) | 95% CI | |
|---|---|---|
| Mild adverse events, n (%) | ||
| Patient sliding down the bed | 16 (25.4) | 14.6–36.1 |
| Venous insufficiency symptoms in lower limbs | 6 (9.5) | 2.3–16.8 |
| Neck pain or low-back pain | 2 (3.2) | 0–7.5 |
| Headache | 1 (1.6) | 0–4.7 |
| Osteoarthritis of the knee | 1 (1.6) | 0–4.7 |
| Bed instability | 1 (1.6) | 0–4.7 |
| Sexual problems associated with the tilting of the bed | 1 (1.6) | 0–4.7 |
| Serious adverse events, n (%) | ||
| Patient sliding down the bed | 4 (6.3) | 3.3–12.4 |
| Venous insufficiency symptoms in lower limbs | 4 (6.3) | 3.3–12.4 |
| Neck pain or low-back pain | 4 (6.3) | 3.3–12.4 |
| Problems related to the bed (instability or breakage) | 3 (4.8) | 0–10.0 |
The table shows the self-reported adverse reactions related to the use of HBE in the sample studied. HBE: head-of-bed elevation.
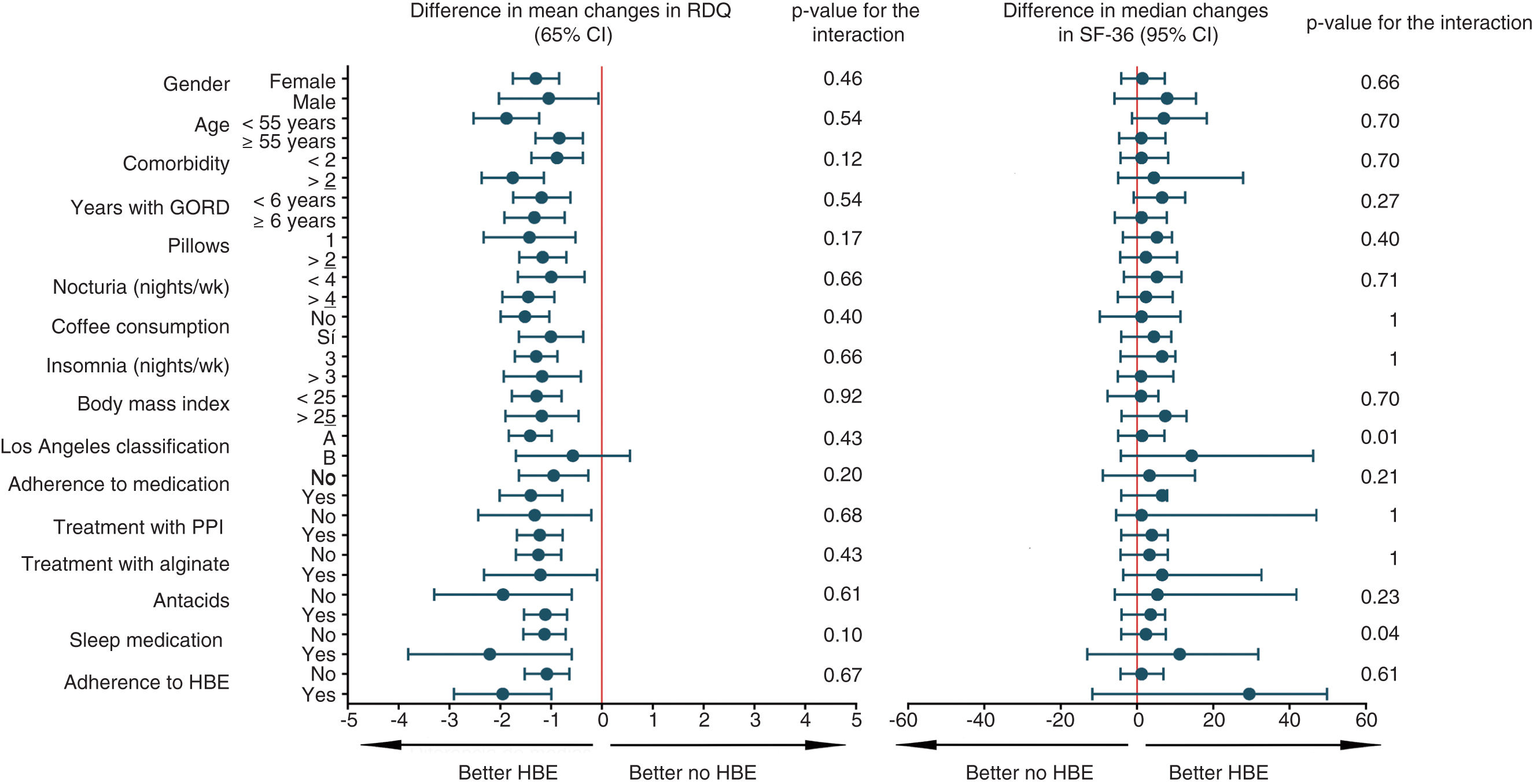
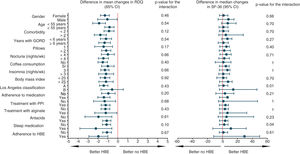
The subgroup analysis for the primary outcome, quality of life, preference and any adverse reaction was univariate and exploratory. Sixteen variables were included and Forest diagrams were constructed to visualise the heterogeneity of the HBE effect, along with statistics of magnitude and interaction. Fig. 3 and Appendix B, Supplementary Figure 5 in the Appendix show all 64 post hoc analyses carried out. Based on this analysis, patients with two or more comorbidities and BMI ≥25 are more likely not to prefer HBE after trying both options. None of the factors that defined the subgroups seemed to increase the risk of any adverse effect (Appendix B, Supplementary Figure 5 in the Appendix). In order to address the problem of multiplicity, we did not formally adjust the p values, but based on the predetermined level of significance and the number of analyses, we estimated that up to three statistically significant interactions (p<0.05) occurred purely by chance.
Subgroup analysis for the primary outcome and quality of life. The figure shows the exploratory subgroup analysis, which shows the mean and median arithmetic differences for the primary outcome and quality of life respectively. HBE: head-of-bed elevation; GORD: gastro-oesophageal reflux disease; PPI: proton pump inhibitor; RDQ: Reflux Disease Questionnaire; wk: week; SF-36: Short Form (36) Health Survey.
As can be seen in Fig. 1, of the 59 patients who used HBE, 17 discontinued the intervention, 15 of them due to adverse effects of the non-pharmacological intervention. As 14 of these 15 patients did not contribute RDQ and SF-36 score information in follow-up, it was not possible to include them from the beginning in the modified intention-to-treat analysis. Because of the risk of this missing information affecting the clinical trial's conclusions, the modified intention-to-treat analysis was performed again using a maximum likelihood model with complete information for missing data. To estimate the effect that the missing scores would have on the main outcomes, auxiliary variables were included in the model which were shown to predict both the observed scores and the absence of such scores, according to published methodology.37,38Appendix B, Supplementary Figure 6 in the Appendix shows that adding missing information to the modified intention-to-treat analysis (n=53), does not change the magnitude or direction of the outcomes of effectiveness and quality of life.
DiscussionIn this study, we found that the use of HBE for six weeks significantly reduced reflux symptoms in patients with GORD and night-time symptoms treated on an outpatient basis with PPI compared to sleeping without that elevation. The magnitude of the effect exceeded the minimal clinically important difference of 10% in the RDQ score which had been pre-specified before the start of the clinical trial, and was consistent in the modified intention-to-treat and per protocol analyses and across most of the relevant subgroups. Similarly, no evidence of a carry-over, period or sequence effect was found that could have affected the validity of the primary outcome.
The result in the primary outcome contrasts with that of a previous multicentre randomised study,39 in which no improvement in reflux symptoms was found with the use of HBE. However, the lack of any effect of HBE on symptoms could be explained by the short follow-up period of two weeks and an HBE magnitude of 15cm, which was less than that used in our study. In the study by Harvey et al.,32 where they used the same HBE height and length of active intervention as in our clinical trial, symptomatic and endoscopic improvement was found with the use of HBE in patients with C–D grade oesophagitis, at a time when PPI had not yet been introduced into clinical practice.40 The above shows the consistency of the effect of HBE in improving reflux symptoms when used as adjuvant therapy for patients already on drug treatment, and can be considered as complementary evidence, given that in the recruitment phase of this study, no patients were found with C–D grade oesophagitis who met the inclusion criteria. The non-randomised clinical studies published to date have also shown an association between the use of HBE and improvement in GORD symptoms.41,42 However, based on the exploratory subgroup analysis for the primary outcome (Fig. 3), the question arises as to whether HBE is really effective in patients not treated with PPI, and perhaps this should be tested in a future clinical trial.
The improvement in the SF-36 quality of life score after the use of the HBE did not reach the magnitude predetermined as clinically relevant of 10% and, as with the primary outcome, we ruled out the possibility that period or sequence carry-over effects might have influenced the result. Exploratory subgroup analysis showed that the effect was generally homogeneous for this outcome. We cannot rule out that this study had a low power to detect differences in quality of life, in view of the fact that there were no previous clinical trials that included this outcome and HBE as a therapeutic intervention from which to estimate important data like the proportion of discordant pairs. This information is needed to calculate the size of the effect of HBE on the variable SF-36, and so adjust the sample size for a marginal homogeneity test such as the McNemar; this being a procedure we were able to perform without any problem for the primary outcome. That said, we cannot rule out the potential for a statistically significant impact of HBE on patient quality of life to be demonstrated in a clinical trial with a larger number of participants.
After testing both sleeping with HBE and sleeping with the standard position, the participants in our clinical trial significantly preferred HBE, which is consistent with what was found in the cross-over clinical trial by Hamilton et al. In that study, after a short period of exposure to experimental therapies of just one night and not reporting the wash-out period, nine out of 15 patients preferred HBE in terms of comfort, when compared to sleeping with a wedge-shaped pillow (proportion: 60%; 95% CI: 35–85; two-tailed p: 0.439).43 We should highlight that the preference found in our study was within the limits of the confidence interval calculated for the preference found by Hamilton et al. Based on the exploratory subgroup analysis, we could suggest the hypothesis of a relationship between good adherence to HBE and greater success of this intervention, perhaps due to better use of non-pharmacological therapy; and this could have led to a greater preference for HBE in these participants (Appendix B, Supplementary Figure 5 in the Appendix). Similarly, there could be an association between being overweight or having more comorbidity and more HBE-related adverse effects, with this possibly influencing these patients to express less preference for the non-pharmacological intervention. However, these assumptions cannot be proven with the evidence from our study and they need to be tested in a clinical trial with a larger number of participants.
Over half the patients in our clinical trial experienced some type of adverse reaction related to the use of HBE and about a quarter of these patients had to stop using the HBE as a result. Interestingly, in only two of the six clinical studies published to date, has any mention been made of adverse effects associated with HBE.32,39,41–44 The Harvey et al. trial reported that two of the 32 patients who used HBE had some adverse effect (sliding down the bed, sexual problems), but these did not lead to discontinuation of the intervention.32 Hamilton et al. only mentioned that, out of a sample of 15 participants, none of them had problems of sliding down the bed due to HBE.43 There are several explanations for the stark contrast in these findings. In our study, we predefined a specific safety outcome for the intervention before the recruitment of patients and the follow-up time was 14 weeks; much longer than that of the other studies cited. Also, each patient had six telephone calls per protocol over the course of the study (4 in the HBE period and 2 in the period with no bed tilting), during which they were systematically asked about adverse reactions. The findings of our clinical trial show the poor tolerance to this non-pharmacological intervention with the degree of tilting tested. We cannot say whether a lesser degree of tilting might minimise adverse effects without compromising the benefits on reflux symptoms.
Within the limitations of this clinical trial, there is the possibility that the measurement of adherence to HBE by serial photographic recording of the wooden blocks in position is not sufficient to rule out discontinuous use of the intervention. However, it was considered impractical and unfeasible from an operational point of view to make surprise visits to each of the participants to verify adherence. Nonetheless, the finding of a more marked preference for HBE in patients adhering to the intervention (per protocol analysis) suggests that compliance with the photographic record could be a good predictor of the proper use of HBE during the clinical trial. There is the possibility of non-differential bias, because changes in patients’ drug treatments were not monitored during the active intervention and wash-out periods. However, the randomisation and crossover nature of the design decrease the likelihood of a possible confusion bias distorting the therapeutic effect of HBE in a targeted way. Additionally, the external validity of this study may have been reduced by the fact that most of the participants were Los Angeles grade A or B. At the same time, that may be seen as an advantage, as there is already evidence of HBE having a therapeutic effect in patients with grade C–D oesophagitis,32 but not for patients with milder degrees of oesophageal mucosa erosion. In terms of the clinical significance of the RDQ and SF-36 score changes associated with the use of HBE, the choice of a change ≥10% as the minimum clinically important difference was purely empirical. However, the patient preference outcome suggests that favourable changes in RDQ score are associated with greater patient preference for the therapy, so the effect on symptoms perceived by participants may have been clinically significant with this cut-off point. Another point to acknowledge is that the timing of the telephone follow-up was not the same in the two groups (HBE and no HBE), as shown in Appendix B, Supplementary Figure 5 in the Appendix. Close telephone follow-up was not considered relevant in patients who were not using HBE, as they were sleeping in a normal, more comfortable position and with less likelihood of adverse effects. This difference in follow-up did not affect the effectiveness or quality of life outcomes, which were measured face to face, but it could have increased the reporting of adverse effects in the group using the HBE due to the larger number of telephone calls. Last of all, addressing the limitation due to missing scores in the modified intention-to-treat analysis, we would simply highlight that our conclusions remained the same, even after adjusting the outcomes with the statistical model used.
In conclusion, HBE as a non-pharmacological adjuvant therapy in the treatment of patients with GORD and night-time symptoms treated with PPI demonstrated a therapeutic effect in reducing reflux symptoms, the magnitude of which was clinically and statistically significant. Although the intervention was not found to have a significant effect on the quality of life outcome, patient preference was consistently in favour of HBE. However, the unexpectedly high percentage of adverse effects casts doubt on whether the net risk-benefit balance for the patient is favourable, and additional studies may be necessary in order to find the least degree of bed tilting that still maintains a therapeutic effect, while minimising the adverse effects of this non-pharmacological therapy.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Villamil Morales IM, Gallego Ospina DM, Otero Regino WA. Impacto de la elevación de la cabecera de la cama en los síntomas de pacientes con enfermedad por reflujo gastroesofágico: estudio aleatorizado simple-ciego (IBELGA). Gastroenterol Hepatol. 2020;43:310–321.