Treatment with rivastigmine, a cholinesterase inhibitor used to treat Alzheimer's disease, is associated with common adverse effects (at least one in 60% of patients1), especially insomnia, nausea and vomiting. However, severe gastrointestinal adverse effects have also been reported, such as upper gastrointestinal bleeding secondary to stomach ulcers.2
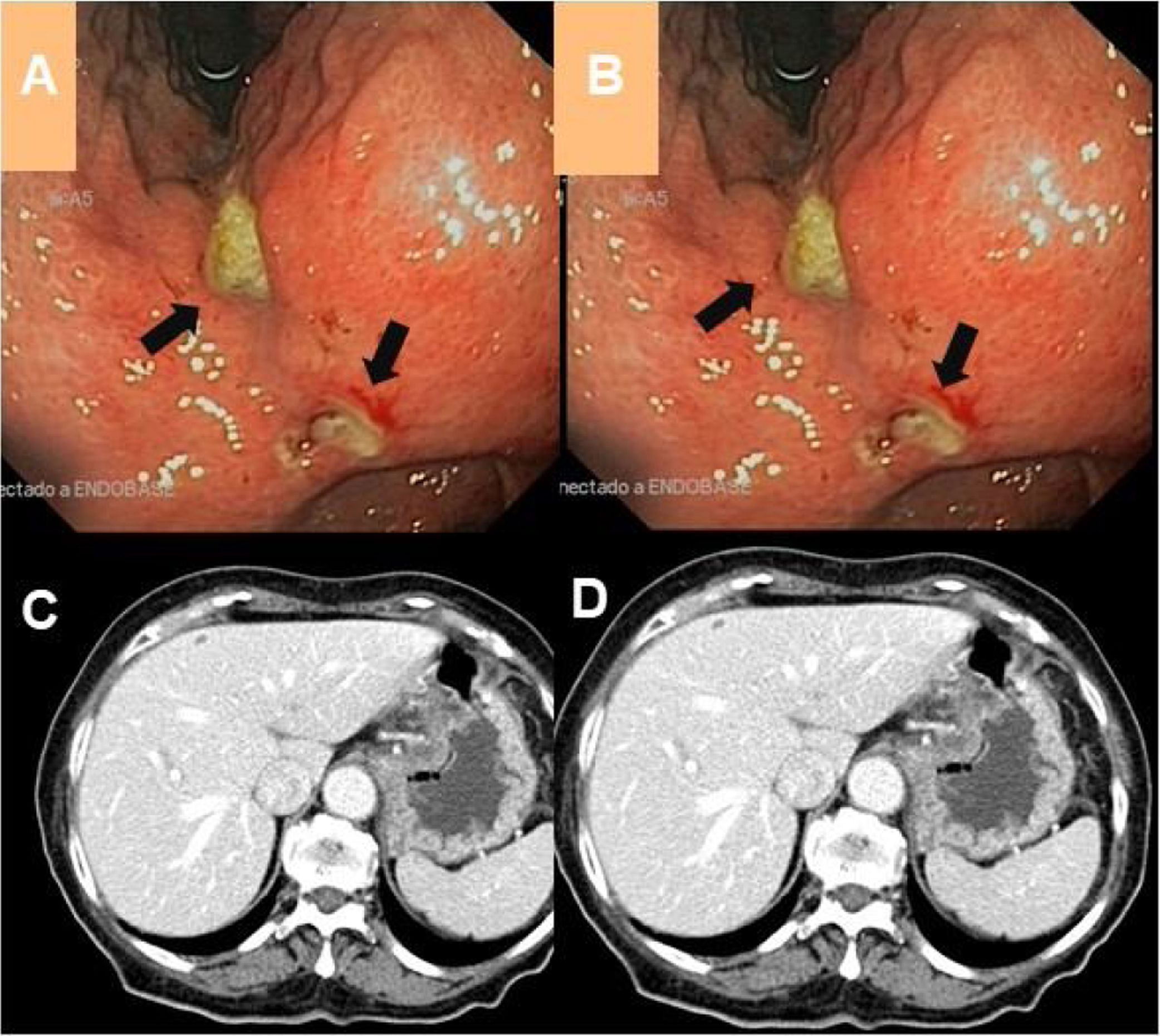
We report the case of an 89-year-old woman with hypertension and recently diagnosed Alzheimer's disease on treatment with rivastigmine. She was subsequently assessed by the gastroenterology department for postprandial vomiting, asthenia, hyporexia and weight loss. Laboratory testing yielded no findings of interest and a gastroscopy was performed (Fig. 1). As a potential malignant aetiology was suspected, multiple biopsies were taken, and a thoracoabdominal computed tomography (CT) scan was done. This did not yield any radiological or histological findings of note.
(A)(B) Gastroscopy images showing, by means of a retroversion manoeuvre, extensive ulceration and development of oedema affecting the lesser curvature of the stomach and the angular incisure (black arrows) spreading towards the antrum. (C)(D) Radiological extension study using contrast-enhanced CT with no notable abnormalities.
Given the suspicion that the patient's signs and symptoms might be secondary to her treatment with rivastigmine, said drug was suspended after agreeing to do so with the neurology department. Two weeks later, the patient's signs and symptoms had resolved, and she had regained her lost weight. In light of the patient's clinical context and the fact that her signs and symptoms resolved after rivastigmine was suspended, another gastroscopy to confirm the regression of the ulcers was ruled out. The patient has remained asymptomatic in follow-up.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: López López S, Prieto Martínez C. Úlceras gástricas múltiples secundarias a rivastigmina. Gastroenterol Hepatol. 2021;44:230–231.