Mammalian target of rapamycin (mTOR) inhibitors, everolimus (EVL) and sirolimus are immunosuppressive agents with a minor nephrotoxic effect, limited to the development of proteinuria in some cases. The combination of EVL and low-dose tacrolimus has proven to be as safe and effective as standard therapy with tacrolimus for the prevention of acute cellular rejection. Early initiation of EVL-based immunosuppressive regimens with reduced exposure to calcineurin inhibitors has been shown to significantly improve renal function of LT recipients during induction and maintenance phases, with comparable efficacy and safety profiles. In patients with established kidney failure, initiating EVL may enable clinicians to reduce calcineurin inhibitors exposure, thereby contributing to the improved renal function of these patients. Although there is not sufficient evidence to recommend their use to prevent the recurrence of hepatocellular carcinoma and the progression of de novo tumours, they are used in this context in routine clinical practice.
Los fármacos inhibidores de la mTOR, everolimus (EVL) y sirolimus, son inmunosupresores con muy poco efecto nefrotóxico, limitado al desarrollo de proteinuria en algunos casos. En la prevención del rechazo agudo EVL combinado con tacrolimus a dosis reducidas tiene una eficacia y seguridad comparables a la inmunosupresión estándar con tacrolimus. La aplicación temprana de una inmunosupresión basada en EVL con minimización de la exposición al inmunosupresor calcineurínico en trasplantados hepáticos permite mejorar los resultados de la función renal, con tasas similares de eficacia y seguridad, tanto en el período de novo como de mantenimiento. En pacientes con disfunción renal establecida la introducción de EVL permite minimizar la exposición al inmunosupresor calcineurínico, con la consiguiente mejoría en la función renal. Aunque no hay evidencia suficiente para recomendar su uso para prevenir la recurrencia del hepatocarcinoma y la progresión de tumores de novo, es práctica clínica habitual utilizarlos en este contexto.
The introduction of the new immunosuppressants has seen the incidence of acute rejection fall and liver transplant (LT) survival rates increase to >80% and >70% at year one and five, respectively.1 However, given that significant toxicity is associated with these drugs, immunosuppressive regimens that meet the following objectives are needed: (1) maintain immunosuppressive efficacy to prevent acute rejection; (2) reduce short- and long-term nephrotoxicity; (3) reduce the number of side effects that give rise to increased cardiovascular risk (diabetes, hypertension, dyslipidaemia, obesity); (4) reduce recurrent hepatocellular carcinoma (HCC); and (5) reduce the onset of de novo tumours. One of the therapeutic strategies to achieve these objectives is based on the withdrawal or reduced administration of calcineurin inhibitors (CNIs) and their combination with mammalian target of rapamycin (mTOR) inhibitors.
Everolimus (EVL) and sirolimus (SRL) act on a different level to CNIs, specifically on the T-cell activation cascade. This inhibits mTOR, the protein kinase involved in the third signal for T-cell activation.2–4
EVL has a shorter half life than SRL,5 enabling stable blood concentrations to be reached more quickly.6
This article is a collection of evidence- and experience-based clinical recommendations proposed by experts in the field.
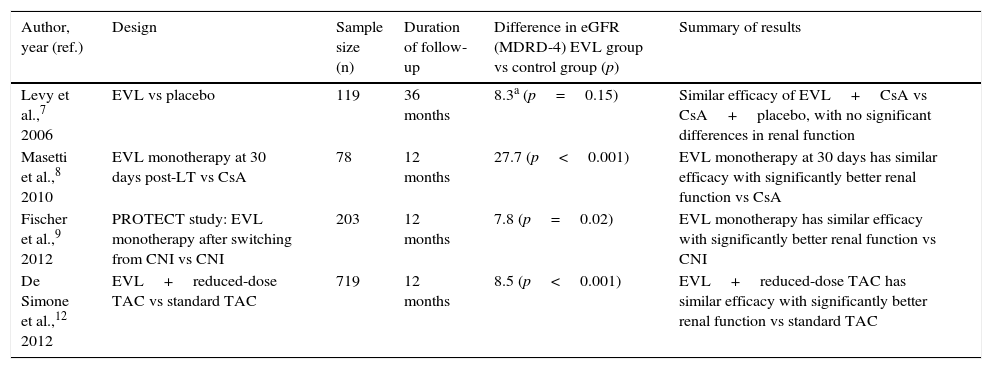
Everolimus in the prevention of acute rejection in liver transplantFour prospective studies with EVL in de novo liver transplant patients have been conducted, each with different objectives (Table 1). Levy et al. analysed the efficacy and safety of EVL up to 36 months after LT in a phase II study (study RAD B158).7 All patients (n=119) were treated with cyclosporin (CsA) plus steroids and three EVL doses were compared (1mg, 2mg or 4mg daily) versus placebo. A trend towards a lower rate of acute rejection was observed in patients treated with EVL versus the placebo group (39.3%, 30%, 29% vs 40%, respectively), which did not reach statistical significance. In another study, Masetti et al. analysed the impact of early withdrawal of CsA (30 days after LT) followed by EVL monotherapy on kidney function in 78 liver transplant recipients.8 No significant differences in efficacy (rejection and survival) were found between the two groups.
Studies on the use of everolimus in de novo liver transplant.
| Author, year (ref.) | Design | Sample size (n) | Duration of follow-up | Difference in eGFR (MDRD-4) EVL group vs control group (p) | Summary of results |
|---|---|---|---|---|---|
| Levy et al.,7 2006 | EVL vs placebo | 119 | 36 months | 8.3a (p=0.15) | Similar efficacy of EVL+CsA vs CsA+placebo, with no significant differences in renal function |
| Masetti et al.,8 2010 | EVL monotherapy at 30 days post-LT vs CsA | 78 | 12 months | 27.7 (p<0.001) | EVL monotherapy at 30 days has similar efficacy with significantly better renal function vs CsA |
| Fischer et al.,9 2012 | PROTECT study: EVL monotherapy after switching from CNI vs CNI | 203 | 12 months | 7.8 (p=0.02) | EVL monotherapy has similar efficacy with significantly better renal function vs CNI |
| De Simone et al.,12 2012 | EVL+reduced-dose TAC vs standard TAC | 719 | 12 months | 8.5 (p<0.001) | EVL+reduced-dose TAC has similar efficacy with significantly better renal function vs standard TAC |
CNI: calcineurin inhibitors; CsA: cyclosporin; eGFR: estimated glomerular filtration rate; EVL: everolimus; MDRD: Modification of Diet in Renal Disease; TAC: tacrolimus.
In the PROTECT 203 study, patients received four-week induction therapy with basiliximab and a CNI with or without steroids.9 From the fourth week, the control group (n=102) continued with the CNI, while the study group (n=101) was administered a reduced dose of the CNI and EVL was introduced with the intention to withdraw the CNI eight weeks after the transplant. No significant differences in survival or biopsy-proven acute rejection were observed at one year,9 and these outcomes were maintained at three and five years of follow-up.10,11
The clinical trial that led to EVL being approved in liver transplant was the phase III study H230412. In this prospective and open-label multicentre study, 719 patients were randomised at 30+5 days post-LT into three arms: tacrolimus (TAC) withdrawal (group suspended early due to a higher rate of acute rejection), reduced EVL-TAC (TAC-r) (EVL at 3–8ng/ml and TAC at 3–5ng/ml) and the control arm, which maintained TAC (at 8–12ng/ml) until month 4 before reducing the dose to 6–10ng/ml. The 12-month global analysis found no inferiority in the incidence of the primary composite endpoint (biopsy-proven acute rejection, graft loss or death) in the EVL-TAC arm, which was lower than in the TAC control arm (6.7% vs 9.7%, p<0.001).12 Efficacy was maintained at 24 and 36 months in patients who continued with EVL.13,14 Based on the results of this study, EVL was approved by the European Medicines Agency and the US Food and Drug Administration in the indication of prophylaxis of acute rejection, in combination with reduced-dose TAC, from the fourth week after liver transplant.
Key points:
- 1.
EVL in combination with reduced-dose TAC has shown comparable efficacy and safety to standard immunosuppression with TAC at 12, 24 and 36 months after liver transplant.
- 2.
EVL in monotherapy is not recommended in the early post-transplantation period owing to the increased risk of rejection.
It is estimated that approximately 50% of liver transplant recipients experience renal dysfunction to varying degrees, with 8–28% suffering severe renal failure. These figures increase as the post-LT follow-up period increases.15–17 However, recent studies have found lower rates of renal dysfunction, with a five-year cumulative incidence of 18–22% using a low-dose CNI.18 A fall >30% in the glomerular filtration rate in the first year after LT has been found to be an important predictor of chronic nephropathy and death after the first year post-LT,19,20 and recent studies suggest a significant increase in mortality when the glomerular filtration rate is <30ml/min/1.73m2 (measured by iothalamate clearance).21
In general, renal function during the first year after liver transplantation is the most important predictive factor of long-term renal failure.15,17 Although CNIs are the cornerstone of immunosuppressive therapy in LT, their use is unequivocally associated with the onset of both acute and chronic nephrotoxicity17,22 and they are considered the main pathogenic mechanism of post-LT chronic kidney disease (CKD).
In light of the above, the late introduction, reduction or even withdrawal of CNIs in both the induction and long-term post-LT maintenance phases should result in greater preservation of renal function. To avoid any detrimental effect on patient survival or liver graft function, these ‘nephroprotective’ protocols should stipulate the use of non-nephrotoxic immunosuppressants like mycophenolate mofetil (MMF) or mTOR inhibitors, potentially in combination with corticosteroids and monoclonal antibodies like basiliximab.
Everolimus in preservation of renal function in the initial post-transplant periodThe first study to assess the early withdrawal of calcineurin inhibitors with early conversion to EVL monotherapy and its effect on renal function was conducted by Masetti et al.8 (see the section “Everolimus in the prevention of acute rejection in liver transplant” and Table 1). In this study, the estimated glomerular filtration rate (eGFR), as assessed by the Modification of Diet in Renal Disease (MDRD-4) equation, was significantly better (87.6±26.1ml/min/1.73m2) in the EVL group versus the CsA group (58.2±17.9ml/min/1.73 m2; p<0.001).
The PROTECT study (see the section “Everolimus in the prevention of acute rejection in liver transplant” and Table 1) was originally published in 2012 by Fischer et al.,9 with the subsequent publication of three- and five-year outcomes.10,11 The objective was to analyse renal function after switching to EVL monotherapy. At one year, renal function assessed by the MDRD-4 equation was significantly better (7.8ml/min/1.73m2; p=0.02) in the EVL group than in the CNI group. At three years, the superiority of the CNI-free regimen increased (9.4ml/min/1.73m2) following the slight but progressive deterioration of the eGFR in the CNI cohort, despite reduced exposure to calcineurin inhibitors.10 The five-year results confirm the superiority trend of the CNI-free regimen in terms of improved renal function, with the eGFR increasing to 11.4ml/min/1.73m2.11 No case of hepatic artery thrombosis was reported.
The registration study that led to the approval of EVL in liver transplant (see the section “Everolimus in the prevention of acute rejection in liver transplant” and Table 1) tested the efficacy of this non-nephrotoxic immunosuppressant in the preservation of renal function after LT. This study compared the results of an EVL+TAC regimen with reduced tacrolimus trough concentrations (3–5ng/ml) vs standard TAC concentrations (8–12ng/ml) until month 4, followed by concentrations of 6–10ng/ml.12–14 The eGFR assessed by the MDRD-4 equation was greater in the everolimus group, with significant differences of 8.5ml/min/1.73m2 and 7.6ml/min/1.73 m2 at 12 and 24 months after transplantation, respectively, versus the control group (p<0.001).12,13 This stabilisation of renal function was maintained at 36 months,14 with no differences in efficacy between both treatment arms. The above results confirm that TAC minimisation combined with the introduction of EVL in the first month after liver transplantation is a safe and effective alternative, with superior renal function compared to standard immunosuppression with TAC. This registration study also showed that the early withdrawal of TAC, substituted with EVL monotherapy, is associated with a significantly greater risk of acute rejection.12
A prospective study comparing EVL (3–8ng/ml) in combination with reduced-dose TAC (<5ng/ml) (EVL+TAC-r) vs standard treatment with TAC (6–10ng/ml) in combination with MMF is currently ongoing in Spain (NCT02040584). The results of this study will reveal the impact on renal function of an everolimus plus TAC-r regimen versus standard immunosuppressive therapy (TAC+MMF).
Everolimus in preservation of renal function in the maintenance periodThe onset of renal dysfunction between three and six months after liver transplantation has been found to be a predictor of long-term renal failure.17,23 Studies on the early post-LT period, as well as kidney transplant studies, suggest that EVL is a potent immunosuppressant that can substantially reduce CNI doses in the early post-LT phase, thereby optimising renal function without impacting on efficacy against rejection.10,24,25
EVL's nephroprotective effect in the post-LT maintenance period has been analysed in numerous studies. In one such study, De Simone et al. found no improved renal function in patients with CNI-related renal dysfunction randomised to convert to everolimus therapy with CNI reduction (20%) or discontinuation (80%) versus the control group.26 The high frequency of dose reductions in the control group (77%) and the relatively long post-transplant time (>3years) might explain the lack of difference in renal function between the two patient groups. However, other non-comparative studies have found a significant improvement in renal function after the introduction of EVL and the reduction or withdrawal of CNIs, without increasing the risk of rejection.27,28 A French multicentre study of 240 patients found a significant mean eGFR increase >8ml/min/1.73m2 (18.2%) in patients with kidney failure assessed 12 months after EVL initiation, at a mean of 4.9±5.2 years after liver transplantation, and 60% did not receive CNIs after this period.26 A recent Spanish observational, retrospective and multicentre study involving 477 patients treated with EVL maintenance therapy found that renal dysfunction was the reason for converting in 32.6% of cases, at a mean of 23.8 months after liver transplantation.29 Although the eGFR, measured by the MDRD-4 equation, improved in all conversion indications (renal dysfunction, HCC prophylaxis and de novo tumours), the greatest improvement in renal function was seen in the group of patients administered EVL due to renal dysfunction, with a significant increase of 10.9 and 6.8ml/min/1.73 m2 at three and 12 months after initiating EVL, respectively. A particularly striking finding of this study was that the improvement in eGFR was greatest in patients who switched early to EVL (within one year of liver transplantation). Indeed, no improvement in eGFR was seen in patients who converted to everolimus five years after transplantation, which suggests that conversion to EVL post-LT should not be postponed unless absolutely necessary, as early CNI minimisation strategies are feasible and safe using EVL. Mean EVL dose and exposure was lower than in the French study28 with no detrimental impact on safety, reflecting the knowledge gained from the increasingly frequent use of this drug.
Key points:
- 1.
The early and personalised implementation of an everolimus-based protocol with minimised exposure to calcineurin inhibitors in liver transplant recipients results in improved renal function with similar efficacy and safety rates.
- 2.
CNI reduction strategies in combination with everolimus effectively prevent renal dysfunction in de novo transplant recipients, as well as during the maintenance phase.
- 3.
In patients with established renal dysfunction, the addition of EVL to the immunosuppressant regimen is a viable option as it minimises exposure to CNIs and may even lead to their withdrawal, thereby improving renal function.
- 4.
The preservation of renal function during the induction and maintenance phases is expected to have an impact on final LT outcomes (morbidity, survival and even cost), particularly in high-risk patients.
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death worldwide30 and accounts for 16–25% of all liver transplants in Spain,31,32 with a post-LT recurrence rate of 16–20%.33 Recurrences are resectable in <10% of cases, and are associated with a mean post-diagnosis survival of one year.34,35 Molecular studies36 have shown that the mTOR pathway is pivotal for HCC development in 30–40% of cases.37 This explains why mTOR inhibitors may have a role to play in the immunosuppressive regimen of HCC transplant recipients.38 This antiproliferative effect on HCC cell lines appears to be maintained even with the concomitant administration of tacrolimus.39 Some studies have established a link between the risk of HCC recurrence and high concentrations of CNI, even in the first month after transplant.40,41
The efficacy of mTOR inhibitors in the prevention or treatment of HCC recurrence after liver transplantation has been analysed in three meta-analyses.42–44 In the first two,42,43 the survival of HCC transplant recipients treated with an mTOR inhibitor as part of an immunosuppressive regimen was superior to subjects not treated with an mTOR inhibitor. However, these results were only based on five retrospective studies with a very limited number of patients, and inconsistent criteria were used to define the tumour characteristics. A more recent meta-analysis44 evinced the same trend in the global analysis, but when the results were adjusted for study and tumour type, a benefit was only observed for those cases which met the Milan criteria.
The results of the SILVER study,45 the only prospective, randomised study to date with extended follow-up that has assessed the use of mTOR inhibitors (SRL) in HCC transplant recipients, were recently published. Globally, the study showed improved recurrence-free survival during the first three to four years of follow-up, which is not maintained from year five. Greater benefits were also found for the subgroup of patients <60 years of age and for those with tumours within the Milan criteria (and not in liver explant high-risk patients). However, the most striking short- and long-term benefits were found in SRL monotherapy patients, although these patients only accounted for 19% of the SRL group. Another limitation of the study was that it did not analyse other recognised poor prognostic factors, whether classification-related (e.g. high risk of recurrence) or patient-related (poor tumour differentiation, microvascular invasion or high alpha-foetoprotein levels).
In a recent study, Yanik et al.46 analysed the effect of SRL maintenance immunosuppression in 3936 HCC transplant recipients between 2002–2012 in the United States of America, 234 (6%) of whom received SRL in the first three months after liver transplantation. The study found no differences in cancer-related death or HCC recurrence between patients who received SRL and those who did not.46 However, the study was limited by the small SRL sample size, and it therefore lacks the statistical power necessary to identify significant differences. Furthermore, 26% of the patients in the SRL group did not undergo follow-up beyond the first three months after transplantation, and explant data are not available. As a result, neither the histological differentiation grade of the tumour nor the potential onset of microvascular invasion were analysed.
The study of the efficacy and adverse effects profile of everolimus in combination with sorafenib in the treatment of post-LT HCC recurrence has yielded contradictory results.47–49
A clinical trial (NCT01888432) evaluating the use of EVL with reduced-dose TAC in living donor recipients is currently ongoing. Its secondary endpoint is to analyse if the benefit of this immunosuppressive regimen for HCC recurrence is maintained in tumours that do not meet the Milan criteria (which is not an exclusion criteria), as well as to assess the role of alpha-foetoprotein, microvascular invasion and tumour differentiation grade.
New studies that prospectively evaluate the advantages of EVL administration in HCC transplant recipients are therefore needed, as such cases represent 30% of this drug's use in clinical practice.29 It is also important to identify the subgroup of patients at high risk of recurrence who could benefit from the use of mTOR inhibitors.
Everolimus in de novo tumoursThe incidence of de novo tumours after liver transplant ranges from 5% to 16%, two to three times higher than in the general population.50 De novo tumours are one of the leading causes of long-term mortality.51 The main risk factors are immunosuppression, age, smoking and alcohol consumption, the latter two taking on particular relevance in de novo lung and oropharyngeal cancer.52,53
Numerous preclinical studies on both in vitro cell cultures and animal models have shown that a wide variety of solid tumours, as well as lymphoproliferative disorders, rely on the mTOR pathway.54 Clinical evidence of the role of mTOR inhibitors in the treatment of post-LT de novo tumours is still scant and based on publications of isolated cases or short retrospective series.55–59 Nevertheless, despite the low level of evidence, one third of all post-LT everolimus is administered in the context of a de novo tumour in routine clinical practice in Spain.29 This is because EVL at concentrations of 3–8ng/ml is administered at many transplant centres in order to minimise or withdraw the CNI.
Despite the lack of sufficient evidence to substantiate a recommendation, mTOR inhibitor-based immunosuppressive regimens to prevent or treat post-LT HCC recurrence, or after the manifestation of a de novo tumour, are routinely used in clinical practice at liver transplant units.
Key points:
- 1.
mTOR inhibitors inhibit angiogenesis and apoptosis to impede the transformation and propagation of neoplastic cells.
- 2.
The retrospective studies performed, as well as the only prospective study carried out to date on HCC transplant recipients, have found lower recurrence and increased survival in certain subgroups of patients who receive mTOR inhibitors. However, until these findings are confirmed in new prospective studies, the generalised use of mTOR inhibitors in all HCC transplant recipients cannot be recommended. Instead, for the time being it seems reasonable to use these drugs in patients at the highest risk of recurrence.
- 3.
The efficacy of mTOR inhibitor-based immunosuppressive regimens to prevent or treat post-LT HCC recurrence, or after the manifestation of a de novo tumour, should be confirmed with future prospective and randomised studies.
As well as the indications mentioned above, mTOR inhibitors may be used in other situations including adjuvant therapy in the event of acute rejection or CNI-induced neurotoxicity even during the early post-transplant period. The EVEROLIVER study29 found that conversion to EVL due to CNI-induced neurotoxicity accounted for 11.6% of cases (56/481), while biopsy-proven rejection accounted for 5.2% (6/481). Nevertheless, there is insufficient evidence to formulate a recommendation concerning these indications.
Practical considerations: metabolism and interactionsEverolimus is an oral drug with a narrow therapeutic index, which is taken twice a day with or without food together with a CNI. As it is primarily metabolised in the liver, the dose must be adjusted in the event of impaired liver function (reduce the starting dose by 30%, 50% and 60% in patients with Child–Pugh A, B and C cirrhosis, respectively).60 Because EVL is metabolised by CYP3A4, the concomitant administration of potent CYP3A4 inhibitors (e.g. ketoconazole, itraconazole, voriconazole, clarithromycin and ritonavir) or inducers (e.g. rifampicin and rifabutin) is not recommended unless the benefits outweigh the risks.
Unlike in a kidney transplant where EVL was administered in combination with CsA, in a liver transplant it was administered with reduced-dose TAC.12 In a kidney transplant, CsA was found to have a synergistic effect with EVL when both drugs were administered together (a lower EVL dose is required when administered in combination with CsA, and, when the CsA dose is reduced, EVL concentrations may also fall,6,61,62 especially if the CsA trough levels are <50ng/ml). This effect was not seen with TAC in a liver transplant, leading to the conclusion that it would seem sensible to administer lower starting doses of EVL with CsA than with TAC in a liver transplant.
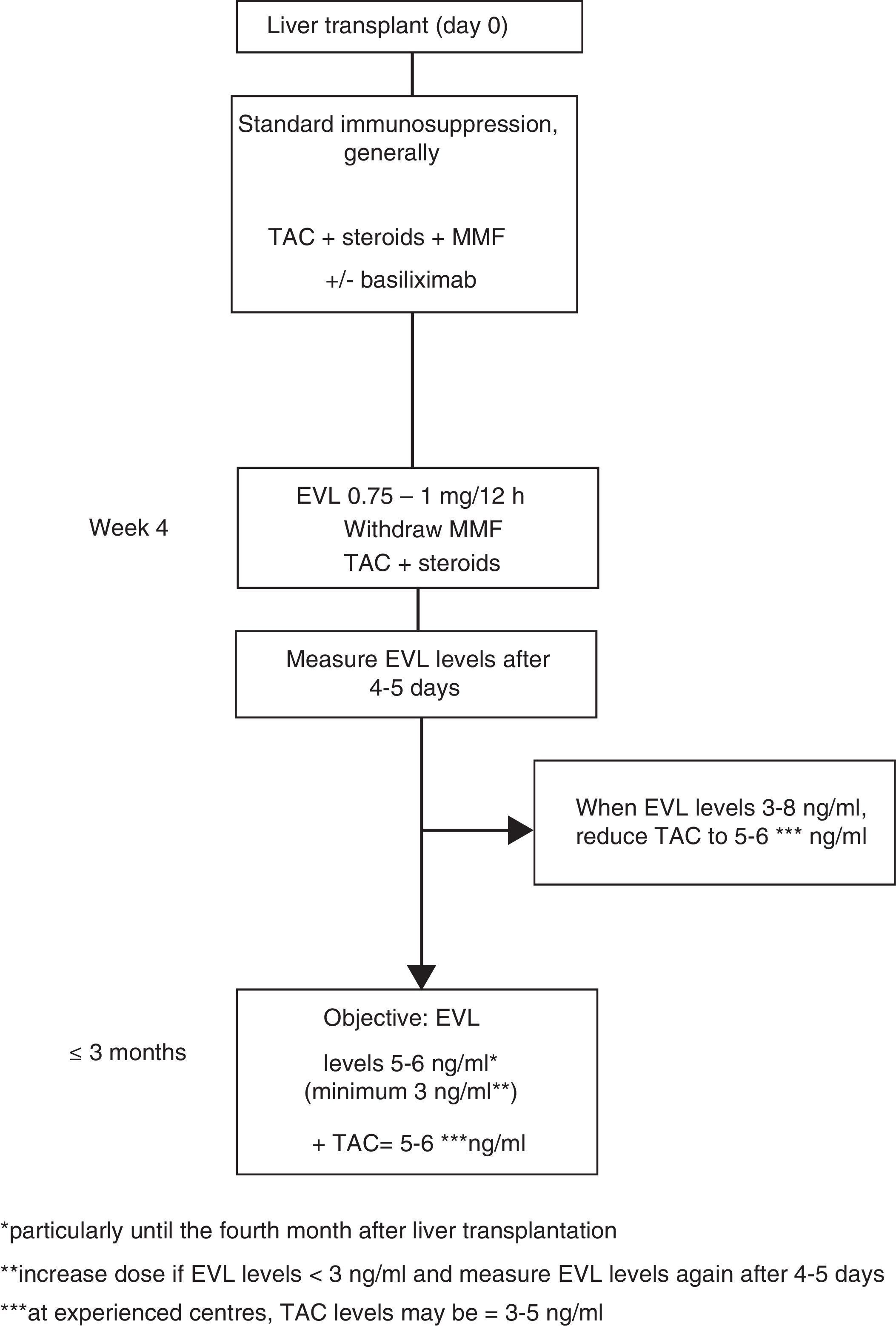
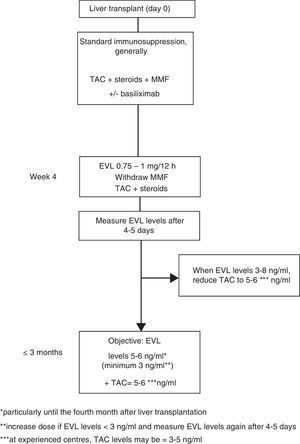
Everolimus dosage63In early use, the EVL starting dose is 1mg every 12h, administered simultaneously with TAC (0.75mg/12h if in combination with CsA). The EVL dose may be adjusted (increased or decreased by 0.25mg/12h) according to trough levels (C0) obtained at least five days subsequent to the last dose adjustment, until a C0 level of 3–8ng/ml is established. Tests should be performed until two consecutive C0 levels show stable EVL concentrations. Once these levels have been achieved, the TAC dose should be progressively reduced. Although the reduced concentration of TAC in combination with EVL has not been established in a de novo liver transplant, 5–6ng/ml may be considered a reasonable dose as this was the mean level established in the registration study.12 However, the design of numerous ongoing clinical trials, as well as the use of EVL in transplant referral centres, suggest that the TAC dose could be reduced still further, to 3–5ng/ml (Fig. 1).
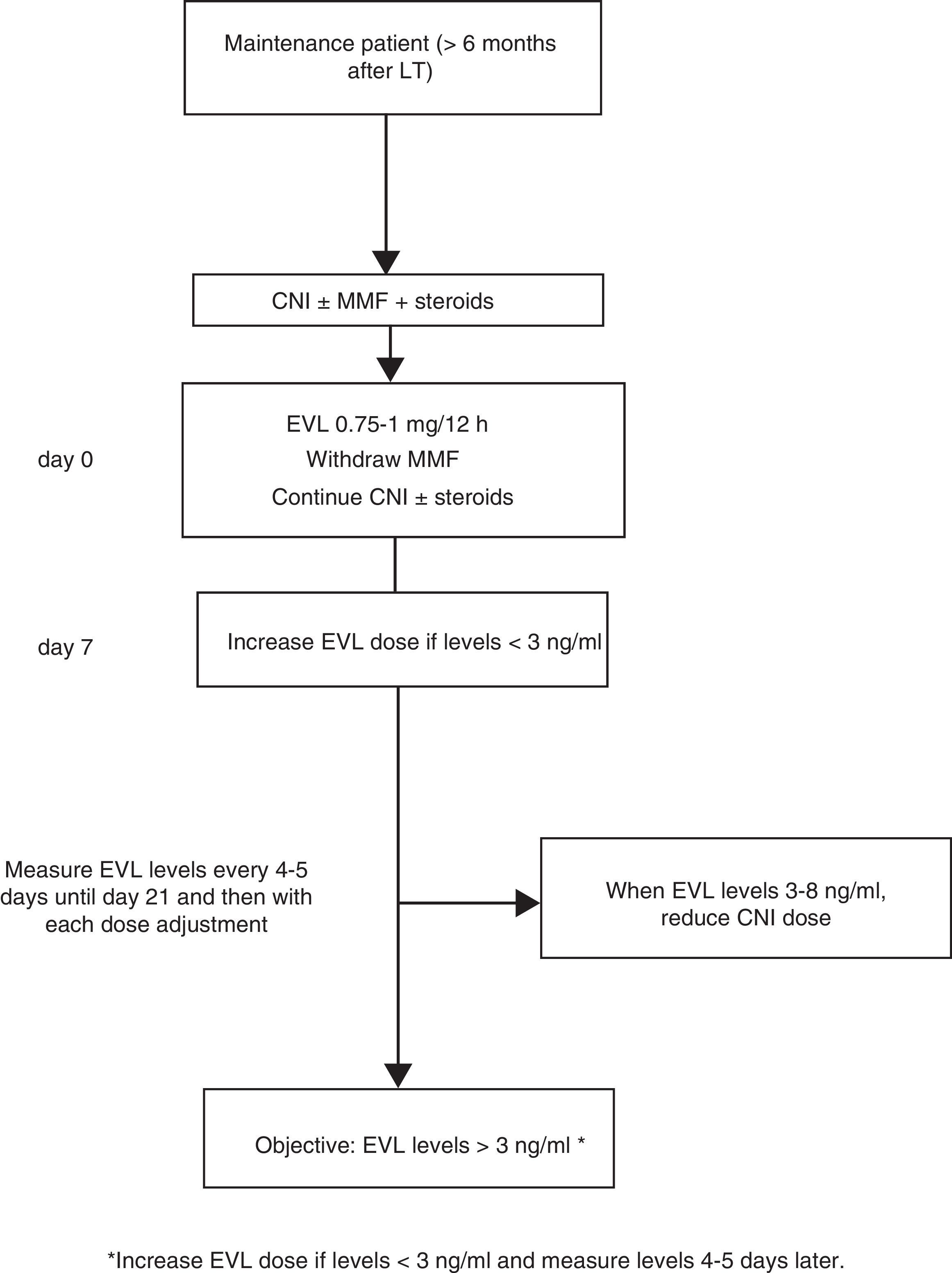
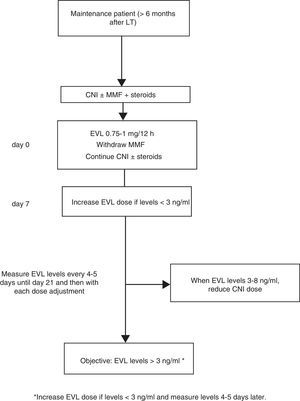
Where EVL administration is delayed, the drug may be administered at a dose of 0.75–1mg/12h while simultaneously reducing the CNI dose by 25–30%, before adjusting the dose in accordance with concentrations and tolerance54 (Fig. 2). The switch to EVL is similar in patients receiving treatment with CsA. However, due to CsA's special interaction with the drug, EVL levels may be reduced if exposure to CsA is significantly reduced (trough concentration <50ng/ml).
Monitoring levelsExposure-efficacy and exposure-safety data have established that EVL C0 levels ≥3.0ng/ml are associated with a lower incidence of biopsy-proven acute rejection than concentrations <3.0ng/ml. The recommended upper limit is 8ng/ml,64–68 as levels >8ng/ml are associated with increased side effects. As such, recommended levels are 3–8ng/ml. Dose adjustment is not required in elderly patients (>65 years) or in patients with renal failure.
EVL levels must be strictly monitored in the following circumstances:
- 1.
Liver failure.
- 2.
The administration of drugs that induce or activate cytochrome P450 CYP3A4 isoenzyme.
- 3.
When cyclosporin-based treatment is minimised.
- 4.
With the onset of adverse effects, in which case the EVL dose should be reduced by at least 0.25mg every 12h, maintaining EVL C0 levels >3ng/ml.
The side effects associated with the use of mTOR inhibitors are well known and can often be controlled with medical treatment and dose reduction.69 The most common EVL-associated adverse reactions recorded by all the controlled clinical trials (CT) are: anaemia, peripheral oedema, elevated creatinine levels when used with full-dose calcineurin inhibitors, hyperlipidaemia, healing abnormalities and oral thrush.12,13,26,70
In the EVL registration study,12 the rate of adverse effects that led to treatment being withdrawn was 25.5% in the EVL-reduced-dose TAC group vs 14.1% in the standard TAC group. Proteinuria (2.9%), recurrent hepatitis C (2%) and pancytopaenia (3%) were the most common events that led to EVL treatment discontinuation.
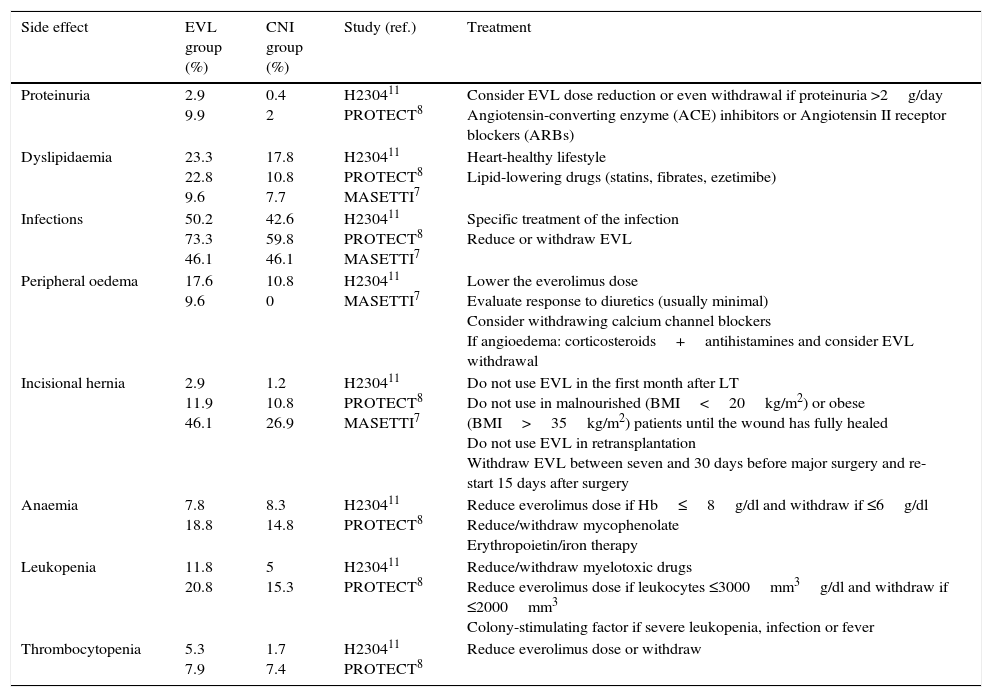
Some of the most common adverse effects together with treatment recommendations are described below (Table 2).
Main adverse effects of everolimus at 12 months in prospective studies.
| Side effect | EVL group (%) | CNI group (%) | Study (ref.) | Treatment |
|---|---|---|---|---|
| Proteinuria | 2.9 9.9 | 0.4 2 | H230411 PROTECT8 | Consider EVL dose reduction or even withdrawal if proteinuria >2g/day Angiotensin-converting enzyme (ACE) inhibitors or Angiotensin II receptor blockers (ARBs) |
| Dyslipidaemia | 23.3 22.8 9.6 | 17.8 10.8 7.7 | H230411 PROTECT8 MASETTI7 | Heart-healthy lifestyle Lipid-lowering drugs (statins, fibrates, ezetimibe) |
| Infections | 50.2 73.3 46.1 | 42.6 59.8 46.1 | H230411 PROTECT8 MASETTI7 | Specific treatment of the infection Reduce or withdraw EVL |
| Peripheral oedema | 17.6 9.6 | 10.8 0 | H230411 MASETTI7 | Lower the everolimus dose Evaluate response to diuretics (usually minimal) Consider withdrawing calcium channel blockers If angioedema: corticosteroids+antihistamines and consider EVL withdrawal |
| Incisional hernia | 2.9 11.9 46.1 | 1.2 10.8 26.9 | H230411 PROTECT8 MASETTI7 | Do not use EVL in the first month after LT Do not use in malnourished (BMI<20kg/m2) or obese (BMI>35kg/m2) patients until the wound has fully healed Do not use EVL in retransplantation Withdraw EVL between seven and 30 days before major surgery and re-start 15 days after surgery |
| Anaemia | 7.8 18.8 | 8.3 14.8 | H230411 PROTECT8 | Reduce everolimus dose if Hb≤8g/dl and withdraw if ≤6g/dl Reduce/withdraw mycophenolate Erythropoietin/iron therapy |
| Leukopenia | 11.8 20.8 | 5 15.3 | H230411 PROTECT8 | Reduce/withdraw myelotoxic drugs Reduce everolimus dose if leukocytes ≤3000mm3g/dl and withdraw if ≤2000mm3 Colony-stimulating factor if severe leukopenia, infection or fever |
| Thrombocytopenia | 5.3 7.9 | 1.7 7.4 | H230411 PROTECT8 | Reduce everolimus dose or withdraw |
Peripheral oedema is a common and at times incapacitating adverse effect, with little or no response to diuretics.
Proteinuria and renal failureIn the clinical trials with post-LT de novo EVL, the rate of proteinuria varies from 3.7–9.9% and is usually mild.8,9,13 The mechanism by which mTOR inhibitors induce proteinuria is not well understood, but is believed to be associated with the reduced tubular reabsorption of proteins. Deteriorated renal function after mTOR inhibitor administration has been reported, particularly in patients with significant prior proteinuria. For this reason, baseline proteinuria must be measured before initiating mTOR inhibitor therapy and its administration is not recommended if concentrations are >800mg/day,71,72 or in patients with eGFR (MDRD4) <40ml/min/1.73 m2. Regular monitoring of renal function and proteinuria (every three to six months) is recommended in patients receiving everolimus.
Incisional hernia and surgical wound complicationsThe use of mTOR inhibitors after a solid organ transplantation has been associated with poor surgical wound healing and the development of lymphoceles. The study by Masetti et al. found a higher incidence of incisional hernia in the EVL group (46.1% vs 26.9%, p=0.16), although it should be noted that everolimus was administered very early, just 10 days after liver transplantation.8 However, in the registration study in which EVL was started one month after LT, the rate of surgical wound and incisional hernia complications was similar between the EVL and non-EVL groups (11% vs 8.3%, p=0.36 and 9.8% vs 7.9%, p=0.52, respectively).12
DyslipidaemiaDyslipidaemia is associated with EVL treatment in both de novo regimens (26.9% EVL group vs 11.6% control group, p=0.001)13 and late conversion regimens (9.7% EVL group vs 2.7% control group p=0.017).24 Dyslipidaemia prevalence ranges from 7% to 43%.73 It is generally controlled with lipid-lowering drugs.
InfectionsThe rate of EVL-associated infections varies depending on the study. Although the rate did not increase in de novo initiation studies,8,13 it did peak when EVL administration was delayed.9,24 Given that mTOR inhibitors inhibit the proliferation of activated T lymphocytes and interferon gamma induced by interleukin-2, both of which are required for immunity against intracellular bacteria, patients may be predisposed to infection by these microorganisms.
In one of the registration studies,13 56% of patients (138/245) from the EVL+reduced-dose TAC group presented with infection vs 51.7% of patients (125/242) from the conventional TAC group, with no significant differences between both groups in either the global analysis or by infection type (bacterial: 19.6% vs 13.2%, viral: 18.4% vs 18.2% and fungal 3.3% vs 6.2%).
Bone marrow suppressionEverolimus is associated with bone marrow suppression that manifests as anaemia (7.8–18.8%), leukopenia (11.8–20.8%) or thrombocytopaenia (5.3–7.9%). Other potential causes of these haematological manifestations, such as viral infections, deficiencies and myelotoxic drugs (MMF, ganciclovir, valganciclovir, etc.), should be ruled out. It can usually be corrected by reducing the EVL dose, although the drug must be withdrawn in some cases.
Stomatitis and aphthous ulcersAphthous ulcers are relatively common in patients treated with EVL. They manifested in 10.6% of patients in the registration study13 and 26.4% of patients in the late conversion studies.26 Concomitant infections must be ruled out in the event of these lesions occurring. Treatment includes reducing the dose of mTOR inhibitors and topical treatment with an anaesthetic. Aphthous ulcers may recur in some patients, requiring EVL to be withdrawn.
Skin lesionsThey generally improve spontaneously within weeks, although topical dermatological treatment and reducing the EVL dose may be necessary. Infection should be ruled out.
PneumonitisIts incidence was recorded as 2–5% in the H230412–14 and PROTECT studies.9–11 Symptoms are variable, ranging from asymptomatic X-ray abnormalities to severe, potentially fatal, respiratory distress. Its prevalence increases when mTOR inhibitor levels exceed the therapeutic margin.
Key points:
- 1.
Renal function should be periodically assessed using the MDRD and/or Chronic Kidney Disease Epidemiology Collaboration equations in patients treated with EVL.
- 2.
Proteinuria should be tested 24hours before introducing EVL and then periodically (every three to six months) once treatment has begun. EVL should be reduced/withdrawn in the event of proteinuria >2g/day.
- 3.
Dose and administration:
- •
Starting dose: from 30 days post-LT at a dose of 1mg/12h orally, administered at the same time as TAC (0.75mg/12h if in combination with CsA), with or without food but always consistent, and at the same time as the CNI.
- •
Maintenance dose and dose adjustment: adjust the dose weekly until trough levels (C0) of 3–8ng/ml are established.
- •
- 4.
Special populations:
- •
No dose adjustment is necessary in elderly patients or patients with renal failure.
- •
Liver failure: mild (reduce the starting dose by 30%), moderate (reduce the starting dose by 50%), severe (reduce the starting dose by 60%).
- •
- 5.
Precautions:
- •
Everolimus trough levels (C0) should be monitored after each dose adjustment and after the administration of drugs that interact with its metabolism.
- •
Renal function (MDRD and/or Chronic Kidney Disease Epidemiology Collaboration equations), proteinuria, complete blood count and lipids should be monitored periodically (every three to six months).
- •
Study funded by Novartis®.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Rubín Suárez A, Bilbao Aguirre I, Fernández-Castroagudin J, Pons Miñano JA, Salcedo Plaza M, Varo Pérez E, et al. Recomendaciones de uso de everolimus en el trasplante hepático. Gastroenterol Hepatol. 2017;40:629–640.
Independent study conducted by experts from the Sociedad Española de Trasplante Hepático (Spanish Liver Transplantation Society, SETH) and endorsed by the SETH. Funded by Novartis.