Esophageal carcinoma is the 7th most frequent type of cancer worldwide, with 572.034 new cases in 2018. The most common histological subtype is the squamous cell carcinoma, although the incidence rate of esophageal adenocarcinoma (EAC) is rapidly increasing.1 The vast majority of EAC arise from Barrett's esophagus located in the lower esophagus and the esophagogastric junction. Less frequently, EAC can develop from the submucosal gland/duct system or gastric heterotopia.2,3
Adenocarcinoma of the esophagus arising from the submucosal gland/duct system is a rare entity, with to our best knowledge, only seven reported cases of patients ranged between 43 and 74 years-old, presenting equally in both sexes.2,3 Tumours were more frequently located in the middle and lower esophagus. Tumors may be more difficult to diagnose, since there is no visible precursor lesion at endoscopy, as in Barrett's esophagus or gastric heterotopia. Patients have been treated with surgery or endoscopic resection, with or without adjuvant therapy. Two patients died at 10 and 15 months. The rest were alive without disease between 18 months and 5 years follow-up.
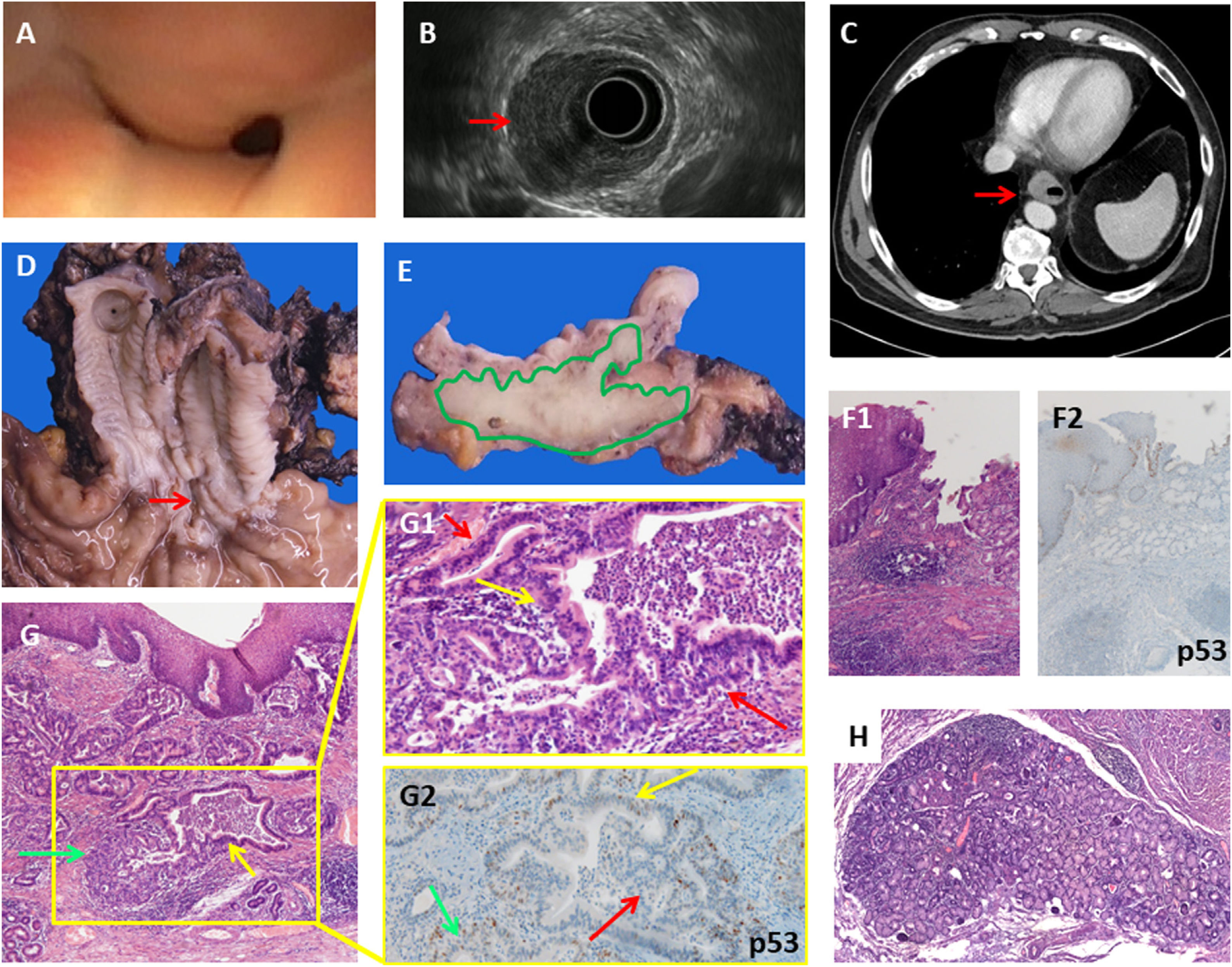
We present a case of a 56-years old male who consulted with dysphagia. The upper digestive endoscopy revealed a distal esophageal stenosis covered with normal mucosa. (Fig. 1A), the Endoscopic Ultrasound (EUS) showed a hypoechogenic lesion within the esophageal wall invading the submucosa, the muscularis propria and reaching the adventitia. However, the mucosal layer was normal on EUS examination (Fig. 1B). EUS-FNA was performed and revealed an esophageal adenocarcinoma. Computed tomography scan (CT) showed concentric wall thickening (Fig. 1C). The patient received neoadjuvant chemotherapy with FLOT (docetaxel, oxaliplatin and fluorouracil/leucovorin) and an Ivor Lewis esophagectomy was performed. Gross examination revealed a normal mucosa with superficial erosion and no Barrett's esophagus (Fig. 1D). Transversal sections showed the tumor located within the wall thickness (Fig. 1E). At histological examination the esophagus was lined with normal stratified squamous epithelium, without intestinal metaplasia at the gastroesophageal junction or heterotopic gastric mucosa (Fig. 1F1 and F2). The tumor was formed by a proliferation of glands invading throughout the esophagus wall, arranged in delimited nests with a tubulo-papillary architectural pattern (Fig. 1G). One definite proof of the submucosal gland origin was the presence of a transition from normal-appearing submucosal glands to cytological atypia and high-grade dysplasia to adenocarcinoma (Fig. 1G1). The tumor was positive for cytokeratin-7 (CK7), CKCAM5.2, CK34βE12, CDX2, carcinoembryonic antigen (CEA), and BerEp4, being negative for CK20 and p53 staining, the latter showing a “wild type”-pattern (Fig. 1G2).
A: Endoscopic view of the stenosis of the distal esophagus covered with normal mucosa. B: Endoscopic Ultrasound (EUS) showing a tumor in the esophageal wall infiltrating the muscular layer (red arrow), but sparing the mucosal layer, and affecting 50% of the circumference. C: CT scan showing mural thickening of the distal esophagus (red arrow). D: Gross findings: a longitudinal erosion in the lower esophagic mucosa (red arrow), with no Barrett's esophagus or any lesions in the rest of the esophageal and gastric mucosa. E: Transversal section of the esophagus wall showing that the tumor (depicted in green) occupies the full thickness of the wall, reaching the adventitia and sparing the mucosa. F1: Microscopic findings: normal esophagogastric junction showing no evidence of Barrett's esophagus (H&E ×10). F2: p53 IHC of esophagogastric junction (×10). G: low power view of the esophageal wall lined by hyperplasic-non dysplastic squamous epithelium. The submucosa and muscular propria show a neoplastic glandular proliferation with normal submucosal glands (green arrow) showing a dilated duct with dysplasia (yellow arrow) (H&E ×10). G1: Higher power view of the inset showing a transition from normal submucosal gland epithelium to dysplasia (yellow arrow) and adenocarcinoma (red arrows) (H&E ×20). G2: “Wild type” p53 staining in a gland showing a transition from normal submucosal gland epithelium (green arrow) to dysplasia (yellow arrow) and adenocarcinoma (red arrow) (p53 IHC ×20). H: Peritumoral hyperplastic submucosal gland without dysplasia (H&E ×10).
The Cancer Genome Atlas (TCGA) characterized EAC finding it more similar to gastric adenocarcinoma than to squamous cell carcinoma of the esophagus, thus, EAC and gastric adenocarcinoma were analyzed jointly.4 Most EAC correspond to the chromosomal instability (CIN) group with mutations in TP53, CDKN2A, CDKN1B, APC (71%) and ERBB2 (13%) genes.4 Little is known about the molecular characterization of EAC arising from submucosal glands, since the cases reported have not been tested. In the present case no mutations were found in any of the 42 genes included in two Next Generation Sequencing (NGS) panels: AKT1, ALK, AR, BRAF, CTNNB1, DDR2, EGFR, ERBB2, ERBB3, ERBB4, ESR1, FBXW7, FGFR1, FGFR2, FGFR3, FGFR3, GNA11, GNAQ, HRAS, IDH1, IDH2, JAK1, JAK2, JAK3, KIT, KRAS, MAP2K1, MAP2K2, MET, MTOR, NOTCH1, NRAS, PDGFRA, PIK3CA, PTEN, RAF1, RET, ROS1, SMAD4, SMO, STK11, and TP53. Furthermore, no copy number alterations were detected in AKT1, ALK, AR, BRAF, CCND1, CDK4, CDK6, EGFR, ERBB2, FGFR1, FGFR2, FGFR3, FGFR4, KIT, KRAS, MET, MYC, MYCN, PDGFRA, and PIK3CA.
The other non-Barrett's associated EAC derive from heterotopic gastric mucosa and is more frequent in males. A third actor to consider is the esophageal gland duct adenoma, located in the lower esophagus, with most published cases being in males between 40 and 60 years old, treated with endoscopic resection and with no recurrence or malignancy during follow up.5 It is uncertain whether esophageal gland duct adenoma can be a precursor lesion of adenocarcinoma. Our case had some hyperplastic benign submucosal glands, larger than normally seen, but it is difficult to state if the tumour was originated in such a lesion, although it cannot be completely excluded (Fig. 1H).
In conclusion, it is important to identify these rare EAC arising in the submucosal gland/duct system, since they could have a different etiopathogenesis and carcinogenetic pathway. Little is known about their genetic profile as no genetic study has been performed in the few reported cases. Our case had a poor response to neoadjuvant therapy, and we did not find any mutation in any of the most frequent genes involved in gastric, esophageal or other frequent human adenocarcinomas.
We are indebted to the Molecular Biology Core from the Biomedical Diagnostic Center (CDB) of Hospital Clinic for technical support. We also acknowledge the support of the Xarxa Catalana de Bancs de Tumors-XBTC, the Banc de Tumors of the Hospital-Clínic-IDIBAPS, and the support of CERCA Programme/Generalitat de Catalunya.