(1) Examine the role of exercise intensity on mental health symptoms in a community-based sample of older adults. (2) Explore the moderating role of genetic variation in brain-derived neurotrophic factor (BDNF) and apolipoprotein E (APOE) on the effects of exercise on mental health symptoms.
MethodThis study is a secondary analysis of a three-arm randomized controlled trial, comparing the effects of 6 months of high-intensity aerobic training vs. moderate-intensity aerobic training vs. a no-contact control group on mental health symptoms assessed using the Depression, Anxiety, and Stress Scale (DASS). The BDNF Val66Met polymorphism and APOE ε4 carrier status were explored as genetic moderators of exercise effects on mental health symptoms.
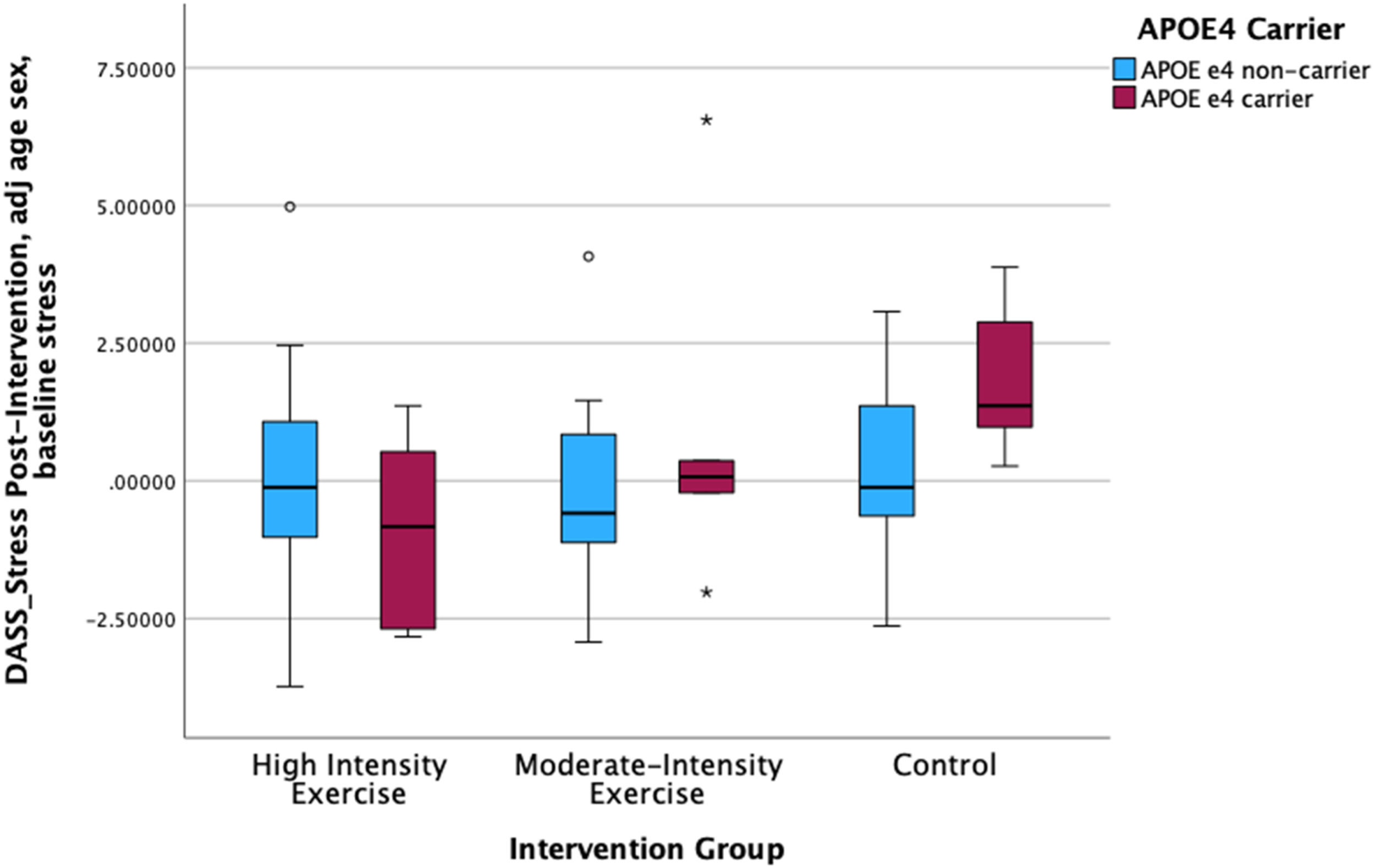
ResultsThe exercise intervention did not influence mental health symptoms. The BDNF Val66Met polymorphism did not moderate intervention effects on mental health symptoms. APOE ε4 carrier status moderated the effect of intervention group on perceived stress over 6 months, such that APOE ε4 carriers, but not non-carriers, in the high-intensity aerobic training group showed a decline in perceived stress over 6 months.
ConclusionsAPOE ε4 carrier status may modify the benefits of high-intensity exercise on perceived stress such that APOE ε4 carriers show a greater decline in stress as a result of exercise relative to non-APOE ε4 carriers.
Physical activity (PA) is beneficial for mental health in older adults in community-based and clinical populations (Landers & Arent, 2007; Paluska & Schwenk, 2000; Rebar et al., 2015). PA is effective as a treatment for late-life psychiatric disorders and as a prevention against clinical depression and anxiety in late-life (Mammen & Faulkner, 2013; McDowell et al., 2019). However, there remains a poor understanding of the dose parameters of PA (i.e., frequency, intensity, duration, type) that may be most beneficial for mental health in aging (Gujral & Oberlin, 2021) and of the moderating factors that may explain variability in the effects of PA on mental health in older adults (e.g., genetic factors, age, sex).
Epidemiological evidence regarding dose-response effects of PA for mental health is inconsistent (Hamer et al., 2009; Hsueh et al., 2021). A meta-analysis of 53 randomized controlled trials found that high-intensity interval training led to improvements in mental wellbeing relative to active and non-active controls and led to improvement in depression severity and perceived stress relative to non-active controls (Martland et al., 2022). One study in depressed adults suggested that both light and vigorous exercise training were more effective at reducing depression severity relative to moderate-intensity PA over 12 months (Helgadottir et al., 2017). Another study in depressed older adults found that high-intensity, compared with moderate-intensity, progressive resistance training was more effective in reducing depression severity over 8-weeks (Singh et al., 2005). Collectively, previous work has focused on clinical samples and suggests various intensities of PA have different effects on mental health symptoms; however, inconsistent findings preclude any interpretation of dose-response effects.
There is clear heterogeneity in PA effects on mental health that is unexplained by previous work. This heterogeneity suggests possible effect moderation, that exercise may work more effectively for some people than others. Possible moderators of PA effects on mental health include genetic factors that influence brain health, including the brain derived neurotrophic factor (BDNF) Val66Met polymorphism and the apolipoprotein (APOE) ε4 allele, both of which have been implicated as genetic variants that elevate risk for depression and cognitive decline (Evans & Rajan, 2015; Pitts et al., 2020; Skoog et al., 2015; Zarza-Rebollo et al., 2022). BDNF and APOE are proteins that are critical for neuro- and synaptic plasticity and prevention of neurodegeneration, and the BDNF Val66Met polymorphism the e4 allele of APOE are genetic variants that adversely effect how these proteins influence cellular functioning in the brain (i.e., in hippocampus), which increases risk for Alzheimer's disease and other related dementias, as well as poor mental health outcomes among older adults (Lim et al., 2015). Previous evidence, however, is mixed and inconclusive regarding whether the mental health benefits of PA are attenuated or accentuated in those with these genetic variants that place them at high-risk for brain health decline (Erickson et al., 2013; Gujral et al., 2014).
The current project is a secondary analysis of a 6-month dose-response trial comparing high-intensity vs. moderate-intensity aerobic exercise vs. a no-contact control condition in a community-based sample of cognitively unimpaired older adults. This paper aimed to examine the role of exercise intensity on mental health (i.e., symptoms of depression, anxiety, and perceived stress) over 6-months and understand whether the BDNF Val66Met polymorphism or carriage of the APOE ε4 allele moderates the effects of exercise on mental health outcomes. We hypothesized that high-intensity training would lead to greater improvements in mental health outcomes relative to moderate-continuous training and the control group. We also predicted that exercise-related benefits for mental health symptoms would be especially pronounced in those with the BDNF Val66Met polymorphism or the APOE ε4 allele.
MethodParticipants and designComprehensive details regarding enrollment, study methodology, and participant characteristics can be found in Brown et al. (2021). This secondary analysis utilized data from the Intense Physical Activity and Cognition (IPAC) study, a single-blind RCT (Australian New Zealand Clinical Trials Registry number: ACTRN12617000643370). We recruited 108 community dwelling adults (of which 99 commenced the study) between 60 and 80 years old who were presently not engaged in regular high intensity physical activity and were not cognitively impaired (>26 on the Montreal Cognitive Assessment and results from the baseline cognitive assessment). All participants provided informed consent per the Human Research Ethics Committees at Edith Cowan University and Murdoch University. Ninety-nine participants completed baseline assessment and were randomly assigned into either high-intensity exercise, moderate-intensity exercise, or control (no exercise) groups using a block randomization protocol. Participants then repeated all assessments within two weeks of completing the 6-month intervention (follow-up).
Mental health outcomesMental health outcomes were assessed at baseline and follow-up with the Depression, Anxiety and Stress Scale (DASS) 21 questionnaire. The DASS has good-to-excellent internal consistency, convergent validity, and discriminative validity and has been validated as an acceptable measure to be used with older adults (Antony et al., 1998; Gloster et al., 2008; Lovibond & Lovibond, 1995).
Self-reported physical activityThe Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire was completed pre- and post-intervention to capture habitual physical activity levels. “Estimated caloric expenditure” was calculated by multiplying the estimated duration per week by the MET (metabolic equivalents) value for each activity and totaling across all applicable activities as kcal/minute.
GenotypingTaqMan genotyping assays were used on DNA extracted from whole blood samples to determine APOE genotype (rs7412, assay ID: C____904,973_10; rs429358, assay ID: C___3,084,793_20) and BDNF Val66Met single nucleotide polymorphism (rs6265, assay ID: C__11,592,758_10). Dichotomous variables indicating APOE ε4 carriers or non-carriers and BDNF Val66Met carriers or non-carriers were created.
InterventionStudy intervention consisted of 6 months of either a high or moderate-intensity cycling program of two 50-minute sessions per week supervised by an accredited exercise physiologist. Exercise intensity was established using the 6 to 20 Borg Scale of Perceived Exertion; such that 6 = no exertion and 20 = maximal exertion (Borg, 1998). The participants allocated to the moderate-intensity group cycled at a constant intensity (50–60 % peak aerobic capacity; 13 Borg Scale) throughout their sessions. Participants allocated to the high-intensity group started sessions at a low-intensity (30–40 % aerobic capacity) then increased to eleven 1-minute high-intensity (>80 % aerobic capacity; 18 Borg Scale) intervals that were separated by 2 min of active recovery (30–40 % peak aerobic capacity). The control group participated in a one-time information session on the positive impact of diet and exercise on aging and cognition without any specific exercise instruction or intervention (Brown et al., 2021).
Statistical analysesAll analyses were conducted using SPSS (version 26; SPSS Inc, Chicago, IL, 2019). We used separate Ordinary Least Squares linear regression models to examine the effect of intervention group on mental health symptoms (perceived stress, depression, anxiety). We used interaction terms (intervention group x BDNF Val66Met carrier / APOE ε4 carrier) to examine the extent to which genetic factors moderated the effect of the intervention on change in perceived stress, depression, and anxiety, in separate models. We adjusted for baseline levels of mental health symptoms (stress, depression, anxiety) in all statistical analyses.
ResultsDuring the intervention, 7 participants withdrew from the original 99 participants that were randomized. An ANOVA found no difference of adherence between the high- and moderate-intensity exercise groups (F (2,66) = 0.80, p = 0.78). However, a lower percentage of the control group (58 % completion) relative to the moderate- (91 % completion) and high-intensity (97 % completion) groups completed post-intervention questionnaires (i.e., DASS; CHAMPS). Participant characteristics can be found in Tables 1 and 2.
Participant characteristics by intervention group at baseline.
| High-Intensitya | Moderate-Intensityb | Controlc | Totald | |
|---|---|---|---|---|
| Age, | ||||
| Mean (SD) | 70.2 (5.3) | 68.4 (4.2) | 68.7 (5.9) | 69.1 (5.2) |
| Gender, n (%) | ||||
| Female | 17 (51.5) | 18 (52.9) | 19 (59.4) | 54 (54.5) |
| Male | 16 (48.5) | 16 (47.1) | 13 (40.6) | 45 (45.5) |
| Years of education | ||||
| Mean (SD) | 13.5 (2.3) | 14.2 (2.4) | 14.5 (2.1) | 14.1 (2.3) |
| APOE ε4 allele carrier status, n (%) | ||||
| ε4 allele carrier | 9 (27.3) | 8 (23.5) | 9 (28.1) | 26 (26.3) |
| BDNF Val66Met carrier status, n (%) | ||||
| Val66Met carrier | 11 (33.3) | 11 (32.4) | 16 (50) | 38 (38.4) |
| MoCA Corrected score | ||||
| Mean (SD) | 26.1 (2.1) | 26.4 (2.8) | 26.7 (2.1) | 26.4 (2.3) |
Note. APOE = Apolipoprotein E; BDNF = Brain Derived Neurotrophic Factor; MoCA = Montreal Cognitive Assessment.
Fitness and self-reported physical activity by intervention group.
| High-Intensitya | Moderate-Intensityb | Controlc | Totald | |
|---|---|---|---|---|
| Intervention Adherence | ||||
| Mean (SD) | ||||
| Baseline | 85.5 (12.5) | 86.3 (9.8) | – | 85.9 (11.1) |
| BMI (kg/m2) | ||||
| Mean (SD) | ||||
| Baseline | 25.8 (3.7) | 26.0 (3.9) | 25.3 (3.4) | 25.7 (3.7) |
| 6 months | 25.4 (3.9) | 26.1 (4) | 24.7 (3.4) | 25.4 (3.8) |
| rVO2max | ||||
| Mean (SD) | ||||
| Baseline | 22.2 (6.3) | 24.7 (6.9) | 22.8 (6.1) | 23.3 (6.5) |
| 6 months | 27.8 (6.3) | 27.5 (7.2) | 22.1 (4.4) | 26 (6.6) |
| CHAMPS "Total Minutes per Week" | ||||
| Range (Median) | ||||
| Baseline | 135–1860 (892.5) | 30–3990 (780) | 105–2970 (705) | 30–3990 (795) |
| 6 months | 90–2880 (960) | 120–3000 (825) | 0–1680 (720) | 0–3000 (840) |
| CHAMPS "Moderate intensity Caloric Expenditure" (kcal/minute) | ||||
| Range (Median) | ||||
| Baseline | 0–4949.5 (1356.1) | 0–14,539.4 (1729.4) | 0–11,968.9 (1517.8) | 0–14,539.4 (1517.8) |
| 6 months | 0–6868.1 (2104.7) | 0–7673.8 (1786.5) | 0–5472.8 (1850.8) | 0–7673.8 (1941.5) |
Note. BMI = body mass index; CHAMPS = CHAMPS Physical Activity Questionnaire for Older Adults; rVO2max = relative maximal oxygen consumption.
n = 33 (Baseline: Intervention Adherence, BMI, rVO2max), n = 32 (Baseline: “Total Minutes per Week”, “Modintensity Caloric Expenditure”), n = 31 (6 months).
n = 33 (Baseline: Intervention Adherence, BMI, rVO2max), n = 32 (Baseline: “Total Minutes per Week”, “Modintensity Caloric Expenditure”), n = 29 (6 months: BMI, rVO2max), n = 28 (6 months: “Total Minutes per Week”, “Modintensity Caloric Expenditure”).
There was no main effect of intervention group on depression, anxiety, and stress symptoms. There were also no group differences between high- and moderate-intensity exercise on mental health outcomes.
We found that the effect of exercise on perceived stress was moderated by APOE ε4 carrier status such that only APOE ε4 carriers in the high-intensity exercise group showed a decline in perceived stress (Fig. 1). APOE ε4 carrier status did not moderate the effect of exercise intervention group on other mental health outcomes. The moderate-intensity exercise and control groups did not show a decline in perceived stress (OLS Regression with reference group of HIT: df= (8, 62) Moderate Intensity x APOE: Beta= 0.259, p = 0.026; Control x APOE: Beta= 0.306, p = 0.013). There were no significant effects for the intervention group x BDNF Val66Met analyses. SeeTable 3, Fig. 1.
Mental Health Outcomes by Intervention Group.
| High-Intensitya | Moderate-Intensityb | Controlc | Totald | |
|---|---|---|---|---|
| DASS: D total score | ||||
| Mean (SD) | ||||
| Baseline | 2.4 (3.0) | 1.6 (2.1) | 1.7 (1.9) | 1.9 (2.4) |
| 6 months | 1.8 (2.2) | 1.7 (2.7) | 1.6 (1.8) | 1.7 (2.3) |
| DASS: A total score | ||||
| Mean (SD) | ||||
| Baseline | 1.4 (1.3) | 1.6 (1.8) | 1.3 (1.3) | 1.4 (1.5) |
| 6 months | 1.9 (2) | 1.4 (1.6) | 1.9 (2.1) | 1.7 (1.9) |
| DASS: S total score | ||||
| Mean (SD) | ||||
| Baseline | 3.7 (2.1) | 3.6 (2.9) | 3.7 (2.7) | 3.7 (2.6) |
| 6 months | 3.3 (2.5) | 3.4 (3.6) | 4.2 (2.8) | 3.5 (3) |
| DASS: D "normal" | ||||
| n (%) | ||||
| Baseline | 27 (87) | 27 (87.1) | 30 (93.8) | 84 (89.4) |
| 6 months | 27 (90) | 24 (88.9) | 17 (94.4) | 68 (90.7) |
| DASS: A "normal" | ||||
| n (%) | ||||
| Baseline | 29 (93.5) | 29 (93.5) | 30 (93.8) | 88 (93.6) |
| 6 months | 26 (86.7) | 25 (92.6) | 13 (72.2) | 64 (85.3) |
| DASS: S "normal" | ||||
| n (%) | ||||
| Baseline | 30 (96.8) | 26 (83.9) | 30 (93.8) | 86 (91.5) |
| 6 months | 29 (96.7) | 24 (88.9) | 17 (94.4) | 70 (93.3) |
Note. DASS = Depression, Anxiety and Stress Scale; D = depression subscale; A = anxiety subscale; S = stress subscale;.
DASS depression subscale score < 7 is “normal”, anxiety subscale score < 6 is “normal” and stress subscale score <12 is “normal”.
Increases in self-reported weekly physical activity (measured by CHAMPS total weekly exercise minutes) over the intervention period was related to a decline in self-reported symptoms of depression on the DASS (F (1, 66) = 4.761, p < 0.033; R2= 0.88). There were no significant interactions of self-reported physical activity change and APOE ε4 carrier status or BDNF Val66Met polymorphism with change in depressive symptoms over the 6-month intervention.
Increases in estimated physical activity-related caloric expenditure over 6 months (measured by CHAMPS estimated weekly caloric expenditure from moderate-intensity physical activity (kcal/minute)) was associated with a decline in depressive symptoms on the DASS (F (1,66) = 7.280, p < 0.009; R2 of 0.12). There were no significant interactions of estimated caloric expenditure and APOE ε4 carrier status or BDNF Val66met polymorphism with change in depressive symptoms over the 6-month intervention.
DiscussionWe conducted a secondary analysis of mental health outcome data from a three-arm randomized controlled trial among community dwelling cognitively unimpaired older adults, comparing the effects of high- vs. moderate-intensity aerobic training vs. a no-contact control group. We also explored the moderating effect of APOE ε4 allele carriage and BDNF Val66Met polymorphism on the relationship between exercise and mental health outcomes. We did not observe an effect of the intervention on any mental health outcomes. However, we found that increases in self-reported physical activity over the intervention period were associated with a decline in depressive symptoms over 6 months across all participants. An exploration of genetic moderators indicated a dose-response effect of exercise (high-intensity > moderate-intensity > control) on perceived stress over 6 months only among those carrying the APOE ε4 allele but no effect of exercise on perceived stress in APOE ε4 non-carriers. The BDNF Val66Met polymorphism did not moderate the effect of exercise intensity on any mental health outcomes.
Moderation of the effect of exercise intensity on perceived stress by the APOE ε4 allele complements the cognitive functioning results from the same pilot trial: only APOE ε4 carriers showed an association between fitness change and change in global cognitive performance over 6 months when the sample was stratified by APOE ε4 carrier status (Brown et al., 2021). Together, the cognitive and mental health results from this pilot trial of high-intensity exercise suggest that individuals at high genetic risk for brain health decline (APOE ε4 carriers) may attain greater cognitive and stress-reduction benefits from high-intensity exercise training relative to APOE ε4 non-carriers, which is consistent with a compensatory hypothesis of exercise-related brain health benefits. These results add to the limited, mixed literature examining whether high-intensity exercise has differential brain health benefits for APOE ε4 carriers vs. non-carriers (Cancela-Carral et al., 2021; Jensen et al., 2019).
The role of the BDNF Val66Met polymorphism in moderating the effects of exercise intensity on cognition and mental health outcomes, respectively, in this pilot trial, were more difficult to interpret. In this secondary analysis, we did not find evidence that the BDNF Val66Met polymorphism moderated the effect of exercise intensity on mental health outcomes, in contrast to the cognitive results from the outcomes reported from the same trial (Brown et al., 2021). The Brown et al. (2021) study based on the same trial showed that only BDNF Val/Val-homozygotes, but not Met-carriers, showed a positive association between fitness improvement due to exercise and improvement in cognitive performance (i.e., global cognition, episodic memory, executive functioning). These findings suggest exercise-related fitness improvement led to cognitive gains only in those a genetic advantage for BDNF signaling in the brain. These results also support the role of BDNF as a potential mediator of exercise-related cognitive benefits. The role of BDNF as a moderator of exercise effects on mental health symptoms is less clearly delineated; the results from our pilot trial suggest that among community-dwelling older adults without clinically significant mental health symptoms, BDNF may not moderate the effect of exercise intensity on mental health symptoms. However, these results need to be replicated in larger, adequately powered trials.
Strengths and limitationsA key limitation to our study findings is the lower completion rate of the mental health outcome questionnaire (DASS) within the no-contact control group relative to exercise groups. Also, given most participants reported subclinical mental health symptoms, lack of findings may be due to limited range of our mental health outcomes. Limited socioeconomic and racial diversity in our sample also limits the generalizability of our findings. Given we were underpowered to test genetic moderators of exercise effects on mental health outcomes, these results need to be validated in larger, adequately powered studies. However, key strengths of our study include the ability to explore possible genetic moderators, a three-arm study design, and high adherence to the exercise protocols.
ConclusionsThe mental health outcome results from this pilot trial suggest that perceived physical activity levels (i.e., if someone “feels” more active) may be more closely linked to depressive symptoms (in an inverse manner) than objective indicators of exercise engagement. An exploratory analysis of possible moderators of PA effects on mental health outcomes among community dwelling older adults indicated that APOE ε4 carriers may especially benefit from high-intensity exercise with regard to reduction in perceived stress. An inverse association between fitness change and change in perceived stress over the 6-month intervention that was present only in APOE ε4 carriers provides further evidence of a dose-response effect of exercise intensity on perceived stress levels among those having genetic vulnerability for neurodegenerative conditions.
FundingThis work was supported by National Health and Medical Research Council Dementia Research Development Fellowship (GNT1097105).