The gut microbiota plays a pivotal role in psychological health, but the mechanistic perspective between gut microbiome and mental health remains poorly understood
MethodThe present case-controlled study recruited 30 unimprisoned subjects and 31 inmates that had been detained in jail for no more than a month. The mental health status, gut microbiota and blood NH3, H2S, 5-hydroxy trptamine and dopamine levels were measured.
ResultsCompared with unimprisoned controls, the fresh inmates exhibited significantly higher scores on anxiety and depression. Both phylogenetic structure and functional genes of the gut microbiota markedly shifted in inmates. Inmates was more Bacteroides-dominated, while unimprisoned subjects were more Prevotella-dominated. Short-chain fatty acids (SCFAs)-producing genera were largely decreased in inmates and were negatively related to mental disorder scores, while Bacteroidetes and Proteobacteria were positive to anxiety and depression scores. Simultaneously, the inmates possessed reduced genes that participate in amino acids, carbohydrates and vitamin cofactors metabolism, but enriched genes that involved in the neurotransmitter-producing Shikimate pathway. Correlation analysis revealed that Anaerotruncus and Prevotella were negative to depression score, and Enterococcus was negative to anxiety score.
ConclusionsOur results revealed potential link between gut microbiota and mental health, leading further support to the microbiota–gut–brain axis theory.
Mental disorders are behavioral, cognitive or psychological syndromes that can cause suffering, distress or even disability (Stein, 2013). Approximately one quarter of people will experience some type of mental disorders in their life time and an estimated 450 million people worldwide currently suffer from mental health problems (Christensen & Pettijohn, 2001). Nowadays, the exact etiologies of most mental disorders are large unknown. Most studies explore the pathology of mental disorders such as depression and anxiety by comparison the phenotypes between clinical patients and healthy persons, but the investigation on the population with subhealth mental status is still sparse.
The gut microbiota plays an important role in maintaining human mental health (Hsiao et al., 2013). Increasing evidence has shown that gut microbes set up a bidirectional communication between the gastrointestinal tract and central nervous system through neural, hormonal and immunological routes, which is called brain–gut axis or microbiota–gut–brain axis (Luna & Foster, 2015). The gut microbial neurochemicals can influence mental health and behavior. For example, as a sensory transducer, serotonin is an important gastrointestinal signaling molecule that utilized by enterochromaffin (EC) cells, and it can activate afferent neurons to transmit signals to the central nervous system (Gershon & Tack, 2007), then affect mental health and behavior. It is estimated that more than 90% of whole body's serotonin (5-HT) and approximately 50% of dopamine (DA) are produced in the gut (Berger et al., 2009). Germ-free mice exhibit decreased concentration of serum 5-HT and elevated serum tryptophan (Wikoff et al., 2009). Clinical studies further indicated that probiotic intervention improved mood and reduced anxiety, depression and other conditions (Bravo et al., 2011). However, it is still unclear how alternation in gut microbiota affects human mental health.
The prison inmates are a special population that is very susceptible to poor mental health such as anxiety and depression. Approximately more than 70% of prisoners have two or more types of mental disorders, and their incidence of mental disorders is 14 times higher than their general population counterparts (Singleton et al., 1998). Although many inmates are with poor mental health, most of them are diagnosed as non-psychiatric and receive no drugs (Shi et al., 2000). In contrast, the patients in psychiatric facilities usually receive many drugs that have profound impact on gut microbiota (Jourova et al., 2016). Therefore, the inmates are less likely to be influenced by drugs, which makes their gut microbiota data more suitable to reflect the real mental status.
In this study, we investigated the mental health and gut microbiota of the non-clinical fresh inmates that are put into jail within a short period. We compared the taxonomic structure of gut microbiota in inmates and unimprisoned subjects, quantified blood concentration of common neurotransmitters and key gut microbial genes that are related to mental health, and identified microbial phylotypes that are associated with poor mental conditions. The results indicated that the microbial community is dramatically altered in inmates and many metabolic genes closely related with mental health status.
Material and methodsParticipants and samplingWe recruited 31 voluntary male persons in jail (inmates/suspects) from the Inner Mongolia Autonomous Region Jail and the Cangzhou Jail of Hebei Province and 30 healthy unimprisoned male subjects who live near the jails. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The inclusion criteria were (i) male; (ii) 20 to 60 years of age; (iii) medically healthy; (iv) no use of antibiotics, probiotics, prebiotics or symbiotic in the six months preceding the study; and (v) consent to blood and fecal sampling. To weaken the influence of lifestyle on the gut microbiota, we chose the inmates who were admitted to the detention center within one month.
Demographic data, eating patterns, food preference, lifestyle and gastrointestinal health of the participants were recorded. Eating patterns and food preference were recorded by a recall questionnaire of food intake in the last 4 weeks. Behavioral and mental health were assessed using the following questionnaires: Aggression Questionnaire (AQ), Self-Control Scale (SCS), Barratt Impulsiveness Scale, 11th Version (BIS-11), State-Trait Anxiety Inventory (STAI), Self-rating Depression Scale (SDS) and Athens Insomnia Scale (AIS). The score of each questionnaire was raw score and calculated by adding up (AIS and SDS) or mean value.
The venous blood (5 ml) was collected from individual and plasma was immediately separated by centrifugation (3,000 g, 10 min) for the quantification of NH3, H2S, 5-HT, DA by commercial assay kit. Fresh fecal samples (5 g) were collected from each person and stored at -80°C until further processing.
Metagenomics analysisFaecal DNA was extracted using a TIANamp Stool DNA kit (QIAGEN Biotech, Beijing, China). Barcoded PCR amplification was performed using a two-step protocol (Wu et al., 2015). Resultant 16S rRNA gene PCR amplicons were sequenced on an Illumina MiSeq platform. Clean sequences after quality control were processed using the QIIME (version 1.7.0) according to the guideline (http://qiime.org/tutorials/tutorial.html). Uclust was used to identify phylotypes and assign sequences to OTUs (Operational Taxonomic Unit) at a distance cutoff of 0.03. The Ribosomal Database Project classifier was used to assign sequences to phylogenetic taxa based on the Greengenes Database. Diversity indices (Chao1, Shannon and Simpson index) were calculated using R 3.2.2. Three nonparametric analyses for multivariate data were used to examine the differences between groups: analysis of similarities (ANOSIM), variance (adonis) and the multi response permutation procedure (MRPP). CCA and PCA were performed using R 3.2.2 with the vegan package V 2.4.0, ade4 package V1.7.4 and plotted by ggplot2 package V 2.1.0. Features of the fecal microbiota specific to mental disorders were determined using a LEfSe method.
The HuMiChip 1.0 functional gene array used in this study targets functional genes at the species/strain levels for the human microbiome. The raw data were uploaded to a microarray data manager pipeline (http://ieg.ou.edu/microarray/) and then preprocessed as previously described (Li et al., 2014).
StatisticsThe results are expressed as the mean ± standard deviation (SD). Statistical analyses were performed using SPSS 22.0. Comparison tests between groups were performed using Chi-square tests for categorical variables and t-tests for continuous variables. Correlations between variables were computed using Spearman's rank correlation. A value of P<0.05 was considered significant. The P values of multiple tests were corrected by FDR.
ResultsThe inmates showed poor mental healthThere was no significant difference between the inmates (Case) and unimprisoned subjects (Control) in general demographics (Supplementary Table S1), diet habits (except more inmates preferred fried food, Supplementary Table S2), and defecation situation (Supplementary Table S3). The behavioral and mental health assessment by questionnaires revealed that the inmates differed significantly from the controls in three of the seven psychological indices: STAI-S, STAI-T and SDS (Table 1), indicating that the fresh inmates had poor mental health including depression and anxiety. However, the disorder scores for STAI-S, STAI-T and SDS in inmates did not reach threshold of depression/anxiety but was in a semi-depression or semi-anxiety status. Blood test showed that the inmates had significantly higher levels of blood 5-HT, DA and NH3 (ammonia) (Table 1). As these neurotransmitters especially 5-HT cannot cross the blood-brain barrier and their blood levels are independent from their brain contents (Kanova & Kohout, 2021), the elevated blood 5-HT and DA in inmates could not indicate a better mental health than the unimprisoned controls.
Statistics for the psychological indices and the blood measures between inmates (Case) and unimprisoned (Control) subjects.
Independent sample t-tests. SD=Standard Deviation. Abbreviations: AQ:Aggression Questionnaire; SCS: The Self Control Scale; BIS-11: Barratt Impulsiveness Scale, 11th Version; STAI-S: State-Trait Anxiety Inventory-State; STAI-T: State-Trait Anxiety Inventory-Trait; SDS: Self-rating depression scale; AIS: Athens Insomnia Scale.
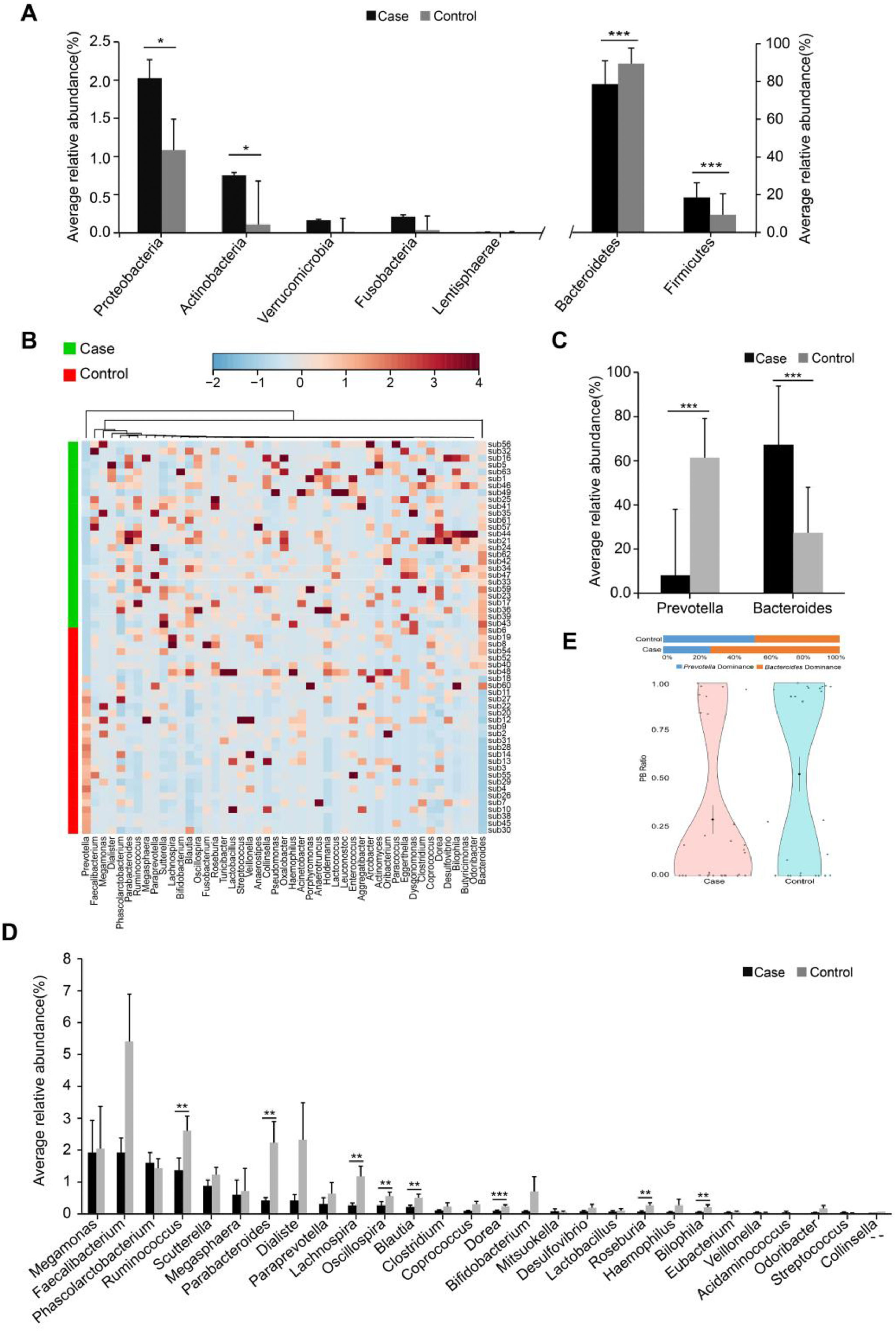
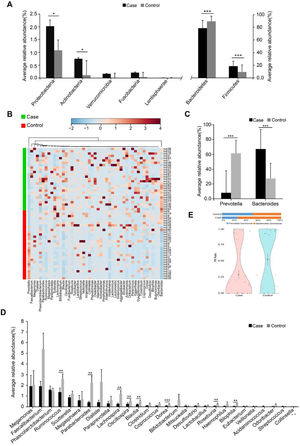
The inmates showed marked shifts in gut microbiota compared with the unrestrained controls. The alpha diversity (Shannon, Simpson and chao1 indices) in inmates was significantly lower than controls (Supplementary Table S4). Dissimilarity tests (Analysis of Similarities (ANOSIM), Adonis and Multi Response Permutation Procedure (MRPP)) also revealed significant difference in the gut microbial structure between the two groups (Supplementary Table S5). The inmate enriched Firmicutes, Proteobacteria and Actinobacteria but decreased Bacteroidetes (Fig. 1A). At the genus level, the putative short-chain fatty acids (SCFAs)/butyrate-producing genera such as Ruminococcus, Lachnospira, Blautia, Dorea, Roseburia and Parabacteroides were significantly lower in the inmates (Fig. 1B and C). Bacteroides was markedly enriched in inmates while Prevotella was more abundant in unimprisoned controls (Fig. 1D). Correspondingly, the Prevotella/Bacteroides (PB) ratio was significantly decreased in inmates (Fig. 1E).
Phylogenetic comparison between inmates (Case) and healthy subjects (Control). (A) The average relative abundance of dominant bacteria at phylum level. (B) Hierarchical clustering analyses of relative abundance at genus level. (C) The average relative abundance of dominant bacteria at genus level. (D) The average relative abundance of Prevotella and Bacteroides. (E) Prevotella / Bacteroides ratio violin plot of two groups. PB= Prevotella / Bacteroides. Black dot with a line in violin plot result were mean and standard deviation. The significance differences between the cases and controls were assessed by MWU test, *P <0.05, **P <0.01 and *** P <0.001, FDR <0.05.
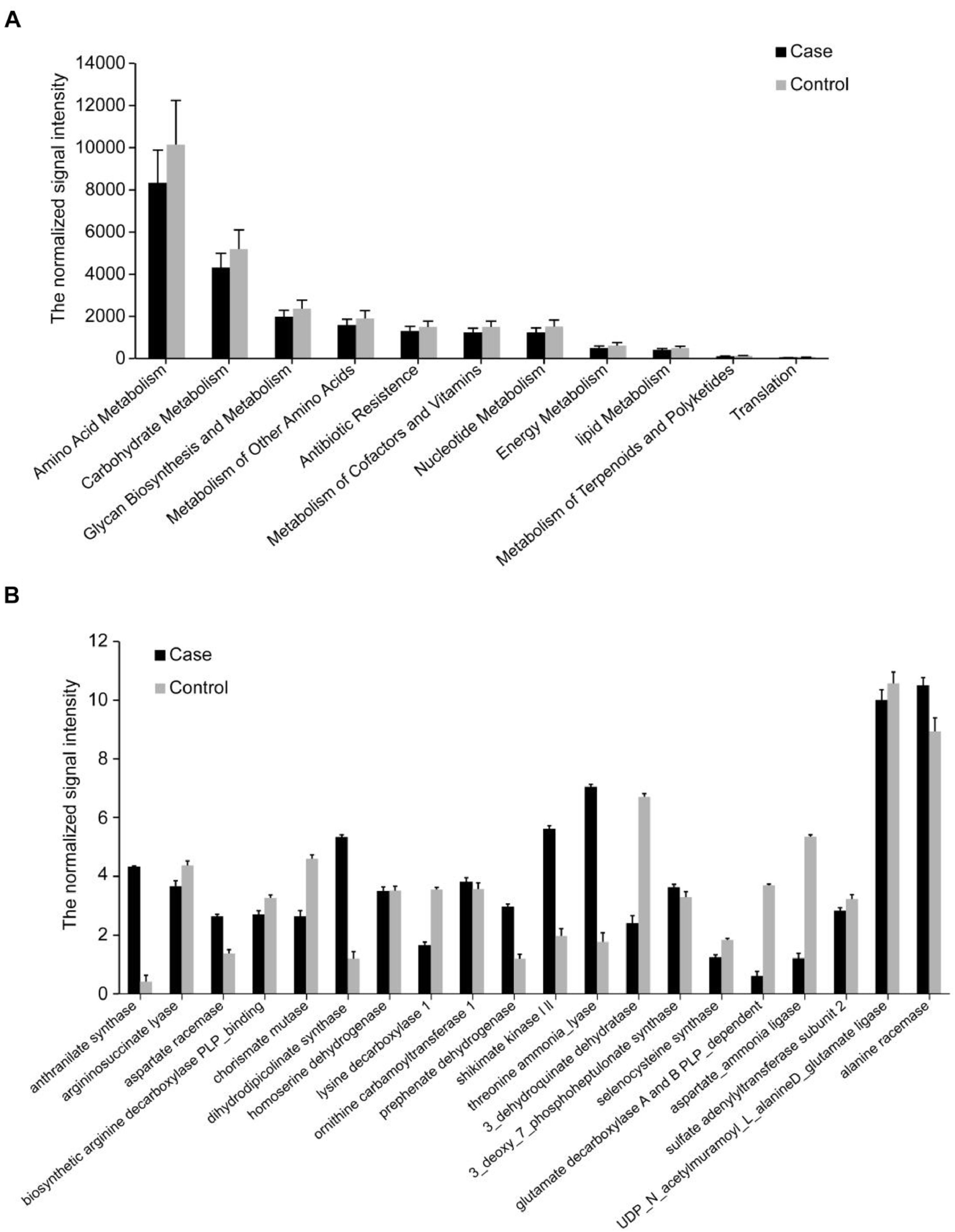
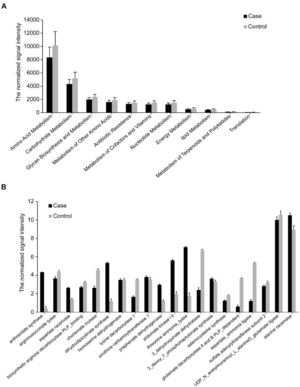
We then analyzed the functional genes via functional human microbiota gene array (HuMiChip) and obtained 20,413 genes from the two groups. The average number of detected genes in each unrestrained control was significantly higher than inmates (11304.20 vs 9434.74, Supplementary Table S6). Similar with phylogenetic results, the inmates showed significantly decreased microbial gene diversity and distinct microbial functional gene structure (Supplementary Table S6). In our previous studies, we found foods could be the most important factor to form gut bacteria and the products of gut bacteria could be important factors impact the mood and behavior, we postulated the hypothesis of Gut-Brain Psychology that emphasizes the foods changing bacteria while the bacteria impacting mood and behavior (Liang et al., 2018). In the present study, we found the overall signal intensities of main metabolic pathways (amino acid, lipid, nucleotides, carbohydrates and energy metabolism) and antibiotic resistance were all decreased in the inmates (Fig. 2A). For individual pathway, most genes showed decreased signal intensity and only a small portion of genes were enriched in the inmates (Supplementary Fig. S1). Typically, the inmate-enriched genes mainly participated in shikimate pathway. The key biosynthetic genes for tryptophan and tyrosine such as shikimate kinase (SK), anthranilate synthase (AS) and prephenate dehydrogenase were increased while chorismate mutase (CM), a key enzyme catalyzes the branch point reaction of phenylalanine and tyrosine biosynthesis to generate prephenate, was decreased (Fig. 2B). These results suggest that the gut microbiota in prisoners has higher potential to produce tryptophan and tyrosine derives, which may result in increased production of 5-HT and DA as indicated by the blood test (Table 1).
Genes involved in metabolic pathways. Only genes displaying significantly different abundances between inmates and controls as assessed by MWU test (FDR<0.05, P<0.05) are shown. The mean signal intensity is presented as the mean ± SD. (A) Genes involved in major metabolic pathways. (B) Genes involved in amino acid metabolism and synthesis.
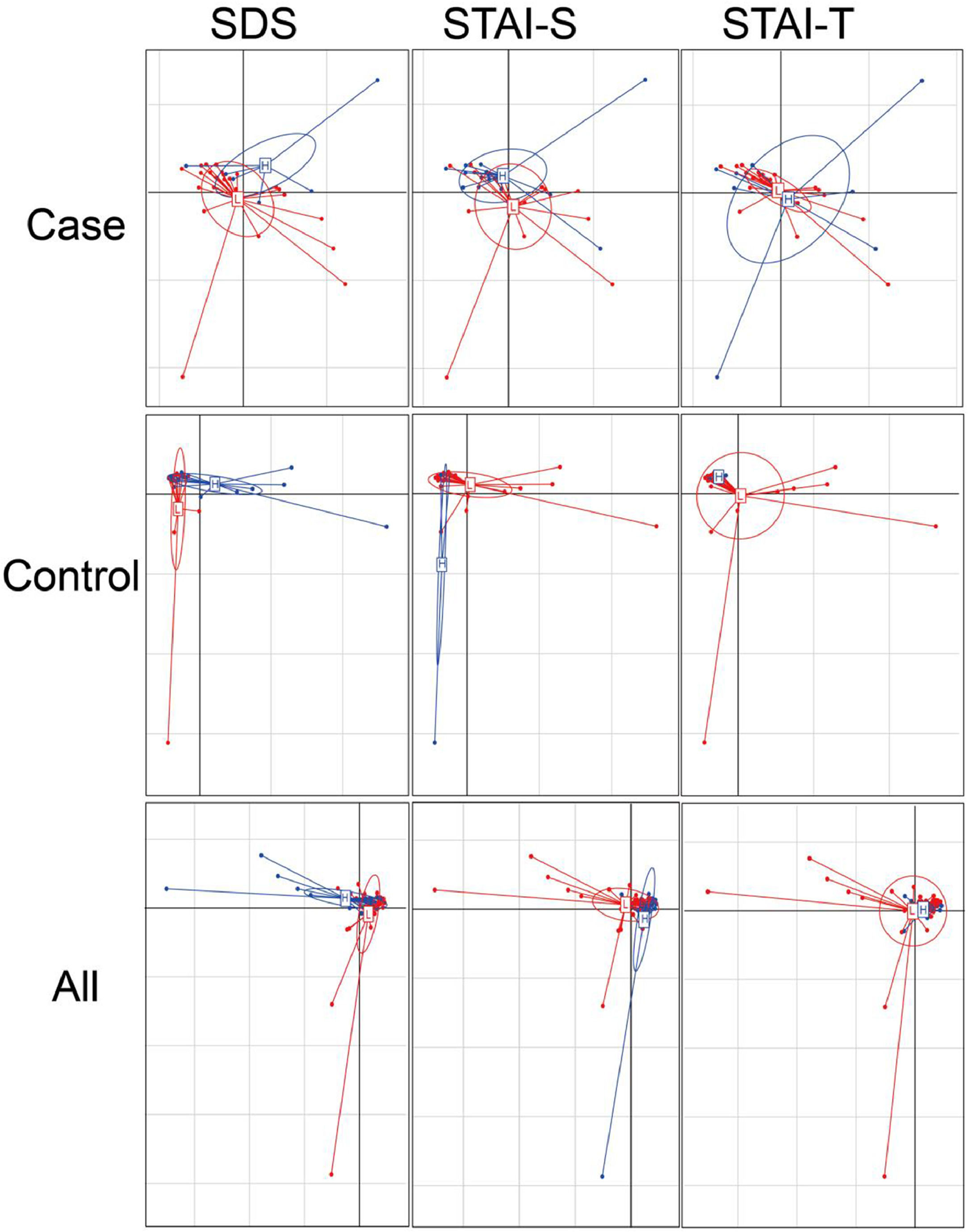
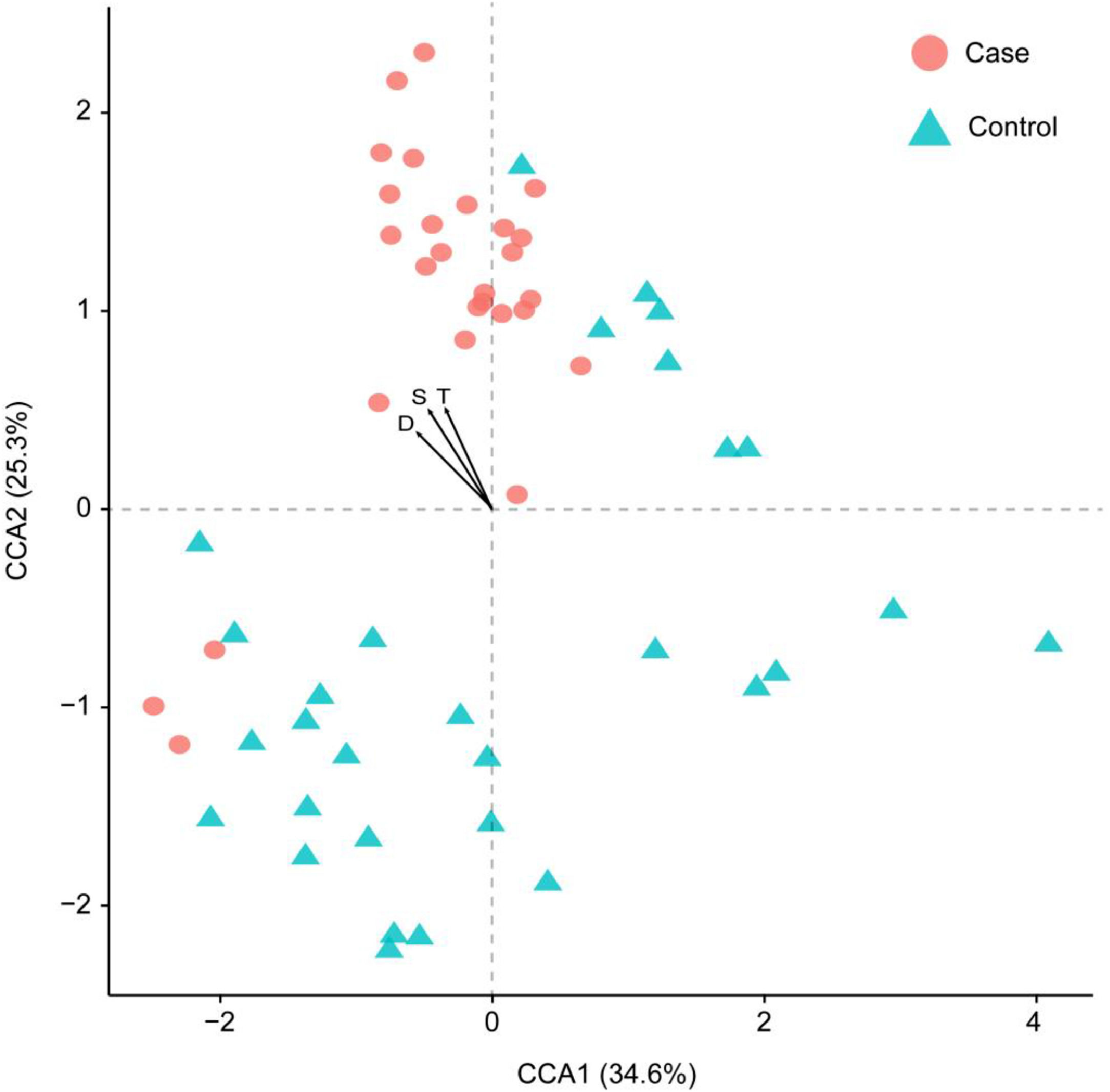
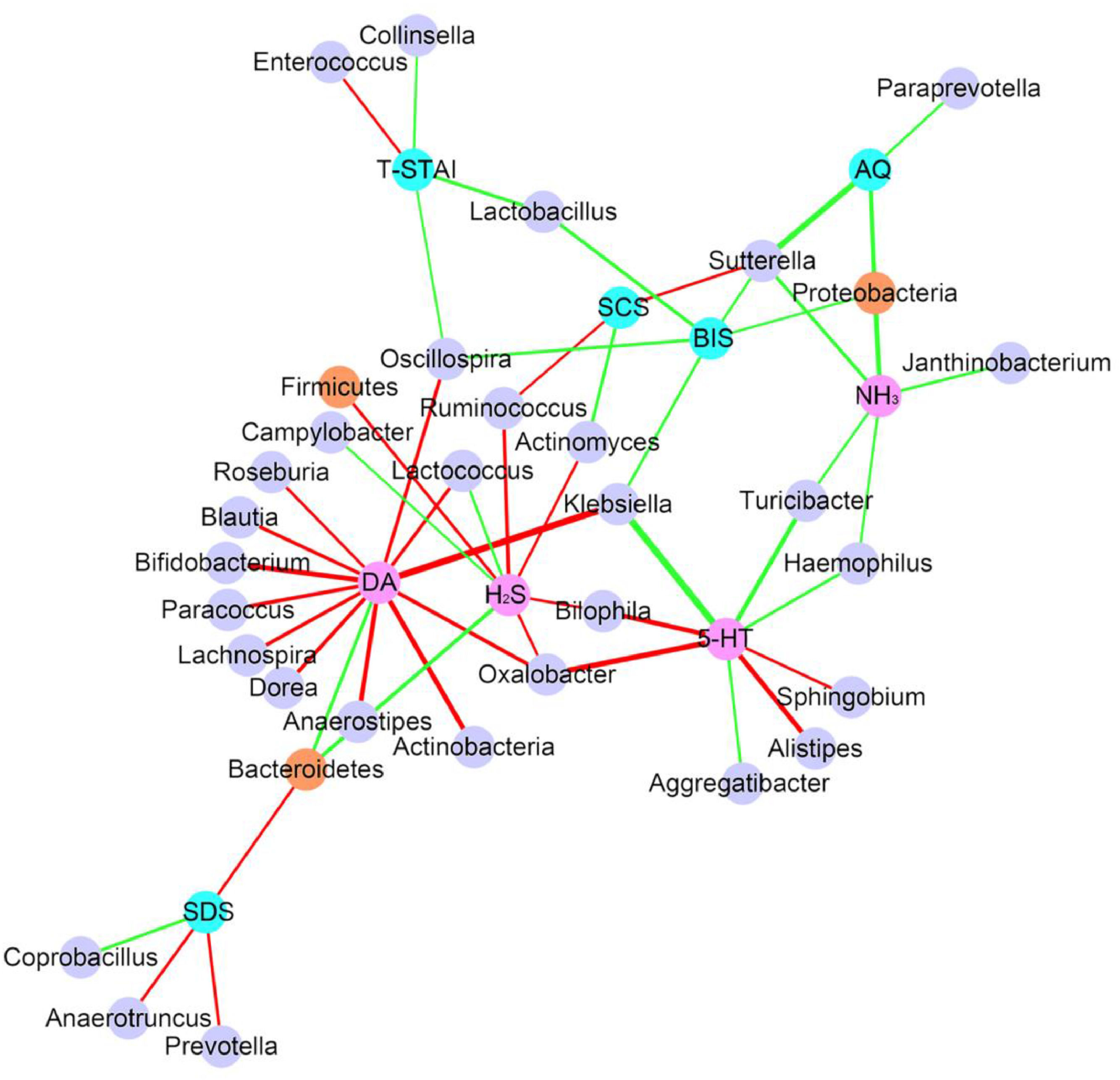
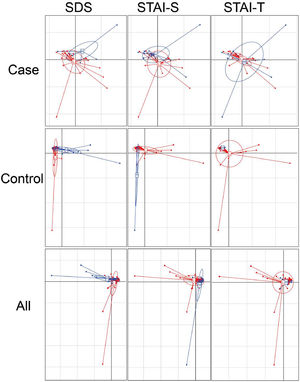
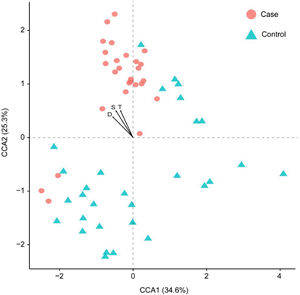
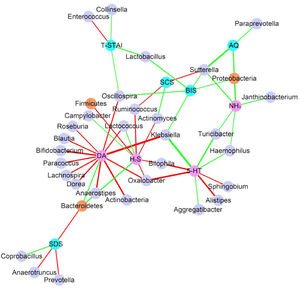
Considering the diet and activity restriction of the inmates, all participants were further divided into two subgroups according to their scores on three psychological indices: STAI-S, STAI-T and SDS. The cutoff scores of these questionnaires were based on Chinese norm score(Dai, 2015). Within inmates (Case), individuals with high SDS or STAI-S scores (H) were generally separated from those with low scores (L) by Principal Component Analysis (PCA), but there was no clear separation between those with high and low STAI-T scores (Fig. 3). For unimprisoned control (Control) and all participants (All), there was no significant separation between individuals with high and low scores on the three questionnaires (Fig. 3). Canonical Correspondence Analysis (CCA) distinguished inmates from unimprisoned controls at the genus level (Fig. 4). The gut microbiota shifted with the changes in mental status, particularly depression (SDS) and anxiety (STAI) (Fig. 4). The spearman's rank correlation network analysis showed that Blood DA was negatively related with SCFAs/butyrate-producing genera such as Roseburia, Blautia, Bifidobacterium, Lachnospira, Dorea, Anaerostipes and Lactoboccus, but positively with Bacteroidetes. Blood 5-HT was negatively related with Alistipes, Sphingobium, Bilophila and Oxalobacter, but positively with Turicibacter, Klebsiella, Aggregatibacter and Haemophilus. Blood H2S was negatively related with Ruminococcus, Firmicutes, Actinomyces, Bilophia and Oxalobacter, but positively with Bacteroidetes and Campylobacter (Fig. 5).
Principal component analysis (PCA) plots of gut microbiota. The x-axis is the first axis and y axis is the second axis. For case, projected inertia of the axis one is 14.28, and the axis two is 11.39. For control, they are 18.14 and 15.72, and 15.31, 12.16 for all subjects. The clustering of the samples were SDS (Self-rating depression scale), STAI-S (State-Trait Anxiety Inventory-State) and STAI-T (State-Trait Anxiety Inventory-Trait) scores within each group and all subjects, based on the genera abundances of gut microbiota. High score (H) and low score (L) groups are shown as blue and red colored circles respectively. For SDS score, the cutoff score is 41 raw score, for STAI-S the cutoff score is 2.5 and STAI-T is 2.6 respectively.
Canonical correspondence analysis (CCA) result showing the first two axes and three main psychological parameters. Samples were clustered based on the relative abundances of each genus. Each sample is represented by a dot, and psychological parameters is represented by an arrow. All samples were scaled down to two dimensions (coordinates) for visualization. S: STAI-S, State-Trait Anxiety Inventory-State; T: STAI-T, State-Trait Anxiety Inventory-Trait; D: SDS, Self-rating depression scale.
The correlation network shows that how microbiota, blood and psychological test results are correlated with each other (Only P<0.05, FDR<0.5 showed). Each node represents an assigned taxonomy name. Brown node represent phylum level and light purple node represent genus level. Pink node represents blood parameters and light blue node represent psychological parameters. Green edges represent positive correlation and red represent negative correlation. Line weight represent correlation coefficient.
Gut microbiota also closely correlated with mental disorder questionnaires. The SDS (Self-rating Depression Scale) score was negatively related with Bacteroidetes, Anaerotruncus and Prevotella but positively related to Coprobacillus (Fig. 5, Supplementary Fig. S2). STAI-T (State-Trait Anxiety Inventory-Trait) score was negatively related with Enterococcus but positively related to Lactobacillus, Oscillospira, and Collinsella (Fig. 5). Other key taxa that were closely related to mental disorders included Proteobacteria which positively related to AQ, BIS and blood NH3 level, Sutterella which positively related to AQ, BIS and blood NH3 but negatively with SCS, and Oscillospira which positively related to BIS and STAI-T but negatively related to blood DA level (Fig. 5).
DiscussionThe gut microbiota plays a pivotal role in psychological health, but the mechanistic perspective between gut microbiome and mental disorders remains poorly understood. In this study, we investigated the potential link between mental disorders and gut microbiota in fresh inmates, a cohort with a sub-healthy mental health status and without drug intervention, to get insights into the correlation of gut microbiota with mental disorders. Our results revealed that the poor mental health of inmates (not depression patients) was accompanied by marked changes in their gut microbiota and functional metagenome profile as compared with unimprisoned subjects, suggesting that gut microbiota may have already changed before obvious mental phenotypes appear and thus may play an important role in the development of depression/anxiety.
The gut microbiota in inmates showed decreased diversity, which was consistent with previous reports that stressor exposure significantly decreased the richness and diversity of the gut microbiota (Nikolova et al., 2021) while treatment with probiotic showed positive psychological effects and was accompanied with increased gut bacterial diversity (Messaoudi et al., 2011). Previous investigations have demonstrated that SCFAs, especially butyrate, were effective to ameliorate depression and anxiety-like behaviors by up-regulating the tight junction protein expression in brain (Tian et al., 2021). In our study, many SCFAs/butyrate-producing genera were significantly lower in inmates than unimprisoned control, which supported the previous report that the depressive disorders were characterized by a reduction of anti-inflammatory butyrate-producing bacteria (Tian et al., 2021).
A striking finding in our study is that Bacteroides was markedly enriched while Prevotella was dramatically decreased in inmates, which led to a large decrease in Prevotella/Bacteroides ratio. It is commonly accepted that the relative abundance of Prevotella, Bacteroides or their ratio are more representative biomarkers of diet, lifestyle and disease status than “Enterotypes” (Gorvitovskaia et al., 2016). Previous studies have shown that Prevotella is correlated with several neurotransmitters production such as gamma-amino-butryic acid (GABA) and tryptophan pathway, thus playing an important role in maintain mental health (Tian et al., 2021). Furthermore, low abundance of Prevotella may result in decreased mucin synthesis and increased gut permeability (Murri et al., 2013), which is closely related to the pathophysiology of chronic depression (Maes et al., 2012). Prior investigations also revealed that the major depressive disorder (MDD) patients commonly enriched Bacteroides (Yang et al., 2020). Therefore, the enrichment of Bacteroides and decrease of Prevotella may contribute to inmates’ poor mental health.
Our results also revealed that gut microbiome is closely related with the blood levels of neurotransmitters and mental health. The gene diversity was largely decreased and most metabolic genes showed decreased signal intensity in the inmates compared with unimprisoned controls. Notably, the key biosynthetic genes for tryptophan and tyrosine (5-HT and DA precursors, respectively) were upregulated in fresh inmates, which may promote the production of 5-HT and DA as indicated by blood test. The spearman's rank correlation analysis between gut microbiota, blood indices and psychological test results showed that SCFAs/butyrate-producing genera such as Roseburia, Blautia, Bifidobacterium, Lachnospira, Dorea, Anaerostipes and Lactoboccus were negatively related with blood DA level. The previous study displayed that SCFAs were significantly lowered and DA metabolism was disordered in children with autism spectrum disorders (ASD) (Wang et al., 2020). Particularly, decreased butyrate may result in poor epithelial barrier integrity, imbalanced of T helper 17/regulatory/T cells and high-degree inflammation (Morris et al., 2017), which may participate in the disturbance of neurotransmitter metabolism in depressive changes (Yang et al., 2021).
Although our work exhibits interesting correlations between gut microbiota and mental disorders, there are some limitations needed to be solved in the future work. First, findings are obtained from a small cohort, more participants should be enrolled to verify the conclusion. Second, the diet structure is relatively simple for inmates as compared to unimprisoned population. Therefore, the correlation between gut microbiota and mental health reported in this paper may not suit for people with highly changeable dietary habits. Last, the blood neurotransmitters levels (5-HT, DA, etc) were higher in inmates with poor mental health, which is inconsistent with many previous reports. The relationship between gut bacteria and individual blood neurotransmitter level needs to be carefully verified, although their correlation with mental health is consistent with general observation.
ConclusionComparison between inmates and unrestrained controls showed that the composition of gut microbiota significantly changed in fresh inmates, a population susceptible to poor mental health but of non-psychiatric. The SCFAs/butyrate-producing genera are markedly decreased and key biosynthetic genes for tryptophan and tyrosine are highly enriched in inmates. The shikimate pathway may be a major metabolic pathway which affects the microbiota-gut- brain axis and ultimately related to host's mental health and behavior.
FundingThis work was supported by China Postdoctoral Science Foundation (2013M541072) and NS Bio Japan, Akita Japan.
Ethics statementThis study was carried out in accordance with the recommendations of The Ethics Committee of the Institute of Psychology, Chinese Academy of Sciences (Beijing, China). The protocol was approved by the Ethics Committee of the Institute of Psychology, Chinese Academy of Sciences (Beijing, China) (permit number H14025). All subjects signed written informed consent voluntarily in accordance with the Declaration of Helsinki.
Data availability statementThe sequence data are available in the GenBank Sequence Read Archive (SRA) database, under the accession number SRP056311.
We are grateful to Prof. Li Wang (Hangzhou Normal University) and Prof. Baoli Zhu (Institute of Microbiology, Chinese Academy of Sciences) for their valuable assistance with this project. We also thank for technique assistances of The Oklahoma Center for the Advancement of Science and Technology (OCAST) through the Oklahoma Applied Research Support (OARS) Project AR11-035.