Background/Objective: This study sought to assess the psychometric properties of the 9-item Shared Decision-Making Questionnaire (SDM-Q-9) in patients with resected, non-metastatic cancer and eligible for adjuvant chemotherapy. Method: A total of 568 patients were recruited from a multi-institutional, prospective, transversal study. Patients answered the SDM-Q-9 after visiting their medical oncologist who, in turn, completed the SDM-Q–Physician version. Reliability, factorial structures [exploratory factor analysis (EFA), confirmatory factor analysis (CFA)], and convergent validity of the SDM-Q-9 scores were explored. Results: SDM-Q-9 showed a clear factorial structure, compatible with a strong and replicable general factor and a secondary group factor, in patients with resected, non-metastatic cancer. Total sum scores derived from the general factor showed good reliability in terms of omega coefficient: .90. The association between patient and physician perception of SDM was weak and failed to reach statistical significance. Males and patients over 60 years of age displayed the greatest satisfaction with SDM. Conclusions: SDM-Q-9 can aid in evaluating SDM from the cancer patients’ perspective. SDM-Q-9 is helpful in studies examining patient perspectives of SDM and as an indicator of the degree of quality and satisfaction with health care and patient-physician relationship.
Antecedentes/Objetivo: Este estudio analiza las propiedades psicométricas del Questionnaire Shared Decision-Making (SDM-Q-9) en pacientes con cáncer resecado, no metastásico y elegible para quimioterapia adyuvante. Métodos: Un total de 568 pacientes fueron reclutados en un estudio multi-institucional, prospectivo, transversal. Los pacientes respondieron al SDM-Q-9 después de visitar a su oncólogo que, a su vez, completó el SDM-Q-versión médico. Se estudiaron la fiabilidad, la estructura factorial (análisis factorial exploratorio y análisis factorial confirmatorio) y la validez convergente de las puntuaciones del SDM-Q-9. Resultados: La escala SDM-Q-9 mostró una estructura factorial clara, compatible con un factor general fuerte y replicable y un factor de grupo secundario, en pacientes con cáncer resecado y no metastásico. La puntuación del factor general mostró una buena fiabilidad en términos de coeficiente omega: 0,90. La asociación entre la percepción del médico y del paciente en la SDM fue débil y no logró alcanzar significación estadística. Los hombres y los pacientes mayores de 60 años mostraron mayor satisfacción con la toma de decisión compartida. Conclusiones: SDM-Q-9 puede ayudar en la evaluación de la toma de decisión compartida desde la perspectiva de los pacientes de cáncer y como indicador del grado de calidad y satisfacción en el cuidado de la salud en la relación médico-paciente.
Recent years have witnessed growing interest in patient participation in shared decision-making (SDM; Elwyn et al., 2014), which represents a shift in traditional forms of healthcare, moving from a paternalistic model to a more collaborative relationship. In this patient-physician alliance, the patient's (and family's) opinion implies the physician's relinquishing part of their control, continued negotiation, and empowering the patient to develop their autonomy (Coulter & Collins, 2011; Schuler et al., 2017). Shared decision includes three essential elements: exchange of information (personal and medical) between patient and physician, deliberation as to diagnostic and therapeutic options, and reaching a consensus (Rodenburg-Vandenbussche et al., 2015; Shay & Lafata, 2015).
The most common cause of patient dissatisfaction is not being duly informed about their medical condition and treatment alternatives (Libert et al., 2017). A survey conducted in eight European countries revealed that most patients wanted to receive more information, as well as to participate more in the decision-making process, although their expectations about their involvement in healthcare decisions differed significantly across countries; for example, in Spain and Poland, patients preferred a more paternalistic model than in Switzerland or Germany (Coulter, Parsons, & Askham, 2008). Likewise, younger people tended to prefer more patient-based communications than older people; this was consistent in all countries (Elwyn et al., 2014).
While great effort is devoted to promoting SDM, it represents an important challenge for physicians (Libert et al., 2017) and the evidence regarding its impact continues to be scarce (Tamirisa et al., 2017). More reliable and valid tools are required to assess SDM's effectiveness and shed greater light on its phases and correlates. The nine-item Shared Decision Making Questionnaire (SDM-Q-9) is a questionnaire designed to probe the SDM process (Kriston et al., 2010). The original version was developed in Germany and based on Elwyn's competences model for patient participation and on additional psychological theories (Kriston et al., 2010; Simon et al., 2006). This 24-item version was reviewed and reduced to a 9-item scale, the SDM-Q-9, which displays excellent internal consistency, high inter-item discrimination and factorial validity (Kriston et al., 2010; Scholl, Kriston, Dirmaier, & Härter, 2015). The SDM-Q-9 has become a commonly used tool for measuring SDM in clinical practice and has been translated into several languages, including English (Kriston et al., 2010; Scholl, Kriston, Dirmaier, Buchholz, & Härter, 2012) and Spanish (De las Cuevas et al., 2015). To date, it has not been applied or validated in cancer patients.
Cancer is a leading public health problem, given its incidence and mortality worldwide (Jönsson, Hofmarcher, Lindgren, & Wilking, 2016). In non-metastatic cancer, surgery and adjuvant chemotherapy can be curative and temporarily impact quality-of-life, due to treatment-related adverse effects or sequelae (Jönsson et al., 2016). Nevertheless, in this context of uncertainty about prognosis and emotional stress, patient-based communication and SDM regarding adjuvant therapy should not only increase patients’ degree of satisfaction, but also their resilience, adherence and tolerance to chemotherapy, and the clinical course of their disease, in addition to mitigating repercussions on their quality-of-life (Libert et al., 2017). Likewise, individualized treatments have proven to benefit cancer patients’ quality-of-life (De Torre-Luque, Gambara, López, & Cruzado, 2016).
The present instrumental study (Carretero-Dios & Pérez, 2005; Ramos-Álvarez, Moreno-Fernández, Valdés-Conroy, & Catena, 2008) attempted to assess the psychometric properties of Shared Decision Making Questionnaire-version patient (SDM-Q-9; Kriston et al., 2010) in Spanish patients with resected, non-metastatic cancer who were eligible to receive adjuvant chemotherapy. The properties assessed were: factorial structure, reliability of the derived scores, and construct validity.
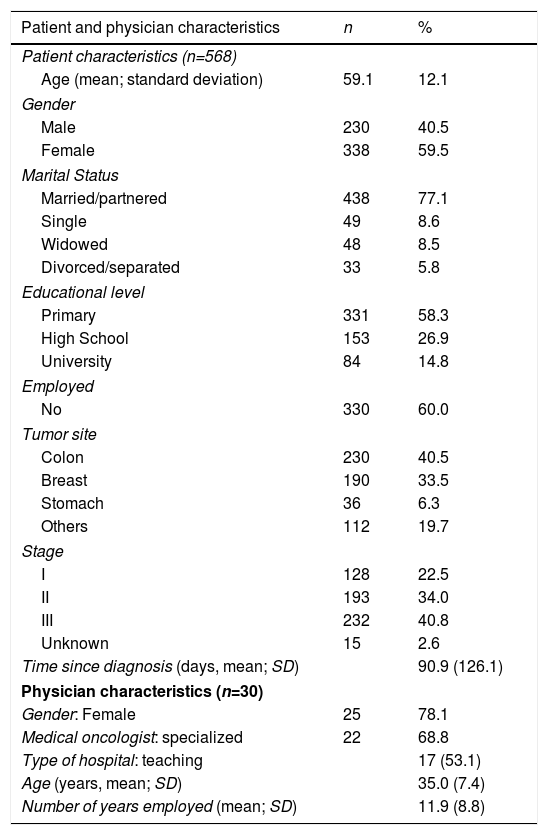
MethodParticipantsThe sample consisted of 568 cancer patients; 59.8% (n=338) were women and the average age was 59.1 years (SD=12.1, range 26-84). Most patients were married or lived with a partner (77.1%) and had completed primary education (58.3%). The most common employment status was retired (60.0%). The sample's clinical characteristics revealed that the most common types of cancer were colon (40.5%, n=230), and breast (33.5%, n=190). All relevant socio-demographic and medical characteristics are included in Table 1.
Patient and physician characteristics.
| Patient and physician characteristics | n | % |
|---|---|---|
| Patient characteristics (n=568) | ||
| Age (mean; standard deviation) | 59.1 | 12.1 |
| Gender | ||
| Male | 230 | 40.5 |
| Female | 338 | 59.5 |
| Marital Status | ||
| Married/partnered | 438 | 77.1 |
| Single | 49 | 8.6 |
| Widowed | 48 | 8.5 |
| Divorced/separated | 33 | 5.8 |
| Educational level | ||
| Primary | 331 | 58.3 |
| High School | 153 | 26.9 |
| University | 84 | 14.8 |
| Employed | ||
| No | 330 | 60.0 |
| Tumor site | ||
| Colon | 230 | 40.5 |
| Breast | 190 | 33.5 |
| Stomach | 36 | 6.3 |
| Others | 112 | 19.7 |
| Stage | ||
| I | 128 | 22.5 |
| II | 193 | 34.0 |
| III | 232 | 40.8 |
| Unknown | 15 | 2.6 |
| Time since diagnosis (days, mean; SD) | 90.9 (126.1) | |
| Physician characteristics (n=30) | ||
| Gender: Female | 25 | 78.1 |
| Medical oncologist: specialized | 22 | 68.8 |
| Type of hospital: teaching | 17 (53.1) | |
| Age (years, mean; SD) | 35.0 (7.4) | |
| Number of years employed (mean; SD) | 11.9 (8.8) | |
Note. n: number, SD: standard deviation, %: percentage.
Patients were recruited by 30 medical oncologists from 14 Spanish hospitals; 78.1% (n=25) of these specialists were female; mean age was 35 years (SD=7.4, range 27-62 years), and 11.9 years of experience in caring for cancer patients (SD=8.8, range 3-37 years). Most were super-specialists (68.8%) working at a public, teaching hospital (53.1%).
InstrumentsSDM-Q-9 is a brief, valid, and reliable questionnaire that evaluates the SDM process from the patient's perspective (Kriston et al., 2010), adapted to Spanish (De las Cuevas et al., 2015). The questionnaire contains nine items, each describing one step of the SDM process (Simon et al., 2006), it was developed to assess the degree to which patients feel involved in the decision-making process. The items are scored from 0 to 5 on a six-point Likert scale ranging from ″completely disagree″ (0) to ″completely agree″ (5). Standard scoring is a simple sum score with values between 0 and 45. Internal-consistency (alpha) reliability estimates are generally high in patients with chronic diseases: .98 (Germany), .94 (U.S.), and .88 (Spain).
SDM Questionnaire-Physician's version (SDM-Q-Doc) is a questionnaire that evaluates the physician's perspective and how well they follow SDM with their patients (Scholl et al., 2012). It was adapted and validated to Spanish (Calderon et al., 2017). The questionnaire consists of nine items, each of which describes one step of the process. The items are scored from 0 to 5 on a six-point Likert scale as “completely disagree” (0) to “completely agree” (5). A simple sum-score with possible values between 0 and 45 is obtained. In this study, Cronbach's alpha for the scale was .90.
SIS is a 4-item scale that was created to ascertain patients’ degree of satisfaction with the information provided by their physician about the disease, risk of recurrence, side effects of treatment, and time dedicated to informing them. The scale provides two subscales: satisfaction with the information provided and satisfaction with the time dedicated. Items are scored from 0 to 4 on a five-point Likert scale ranging from ″completely disagree″ (0) to ″completely agree″ (4); the higher the score, the greater the satisfaction with the information provided. The scale revealed a Cronbach's alpha value of .82 in our study.
The patients’ medical and demographic variables included were: age, gender, marital status, educational level, occupational sector, tumor site, stage, and time since diagnosis. The oncologist-related variables included age, gender, years of experience, area of specialization (general (treating all kinds of tumors) vs. super-specialized (treating one specific subtype of tumor) and type of hospital (academic vs. non-academic).
ProcedureThis is a multi-institutional, prospective, transversal, observational study that pooled consecutive patients recruited at 14 Spanish teaching hospitals from June 2015 to May 2017. The study is part of a research program investigating patients with cancer; it is funded by the Continuous Care Group of the Spanish Society of Medical Oncology (SEOM). The study was approved by the Ethics Review Board at each institution and by the Spanish Agency of Medicines and Medical Devices (AEMPS). Inclusion criteria were being ≥18 years of age, having a histologically confirmed, non-advanced, solid tumor surgically treated for which international clinical guidelines consider adjuvant treatment to be an option. Patients with metastatic disease, treated with preoperative radio- or chemotherapy, or with adjuvant hormonal or radiotherapy without chemotherapy were excluded. Similarly, physical ailments, comorbidity, and/or age precluding chemotherapy, and personal, psychological, family, sociological, geographical, and/or underlying medical condition that, in the investigator's opinion, could hinder the individual's ability to participate in the study were also cause for exclusion, since these patients did not have to decide on adjuvant therapy. The evaluation was performed in all cases approximately one month following surgical resection, in the context of patients’ first visit with the oncologist to decide on adjuvant chemotherapy. Data collection procedures were similar at all hospitals. Participation was voluntary, anonymous, and would not affect their care in the slightest. The participants completed the questionnaires individually, with no limit on time. Data were collected and updated by medical oncologists, specifically trained to comply with the study requirements, via a web-based platform (www.neocoping.es). Of the 627 patients screened, 59 were not eligible (17 did not meet inclusion criteria; 23 met exclusion criteria and 19 had incomplete data).
Data analysisDescriptive analyses were conducted for every SMD-Q-9 item and explored means standard deviations and distributions of the item scores. To assess the factorial structure of the scale, the sample was randomly split into two groups and different Exploratory Factor Analysis (EFA) solutions based on previous reported results were performed on the first split-half sample. The adequacy of the inter-item correlation matrix to be factor analyzed was first assessed using the Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy. The different hypothesized solutions were next fitted by using robust, unweighted least squares estimation with mean-and-variance corrected fit statistics as implemented in the FACTOR program (Lorenzo-Seva & Ferrando, 2013). They were: (a) unidimensional (as the scale was initially designed to be single-trait), (b) unidimensional with item 1 omitted (De las Cuevas et al., 2015) and (c) bidimensional with correlated factors. From the EFA results, a simple and clearly interpretable bifactor structure (Lorenzo-Seva & Ferrando, 2013) could be specified. This structure was next fitted to the entire sample with FACTOR by using the same estimation procedure described above.
In all the tested solutions above, the goodness-of-fit indices used to assess model-data fit were: (a) RMSEA, with its 95% confidence interval (as a measure of approximate fit); (b) Goodness-of fit-index (GFI), and (c) the root mean square of the standardized residuals (z-RMSR), (as absolute measures of fit), and (d) the comparative fit index (CFI), (as a relative measure of fit with respect to the null independence model). We followed the usual rules in deciding model appropriateness (Schermelleh-Engel, Moosbrugger, & Müller, 2003). In addition to model-data fit measures, additional indices of appropriateness for assessing the strength and replicability of the solution (H index) as well as closeness to unidimensionality (ECV index) were also obtained (Ferrando & Lorenzo-Seva, 2017).
Once the proposed structure had been fitted and found appropriate, scores based on this structure were obtained and their reliability was assessed by using the omega coefficient (McDonald, 1999). Finally, construct and external validity were assessed on the basis of these scores by using product-moment correlations and univariate ANOVA-based mean-group comparisons using Bonferroni corrections. For all the tests conducted, bilateral statistical significance was set at p≤.05.
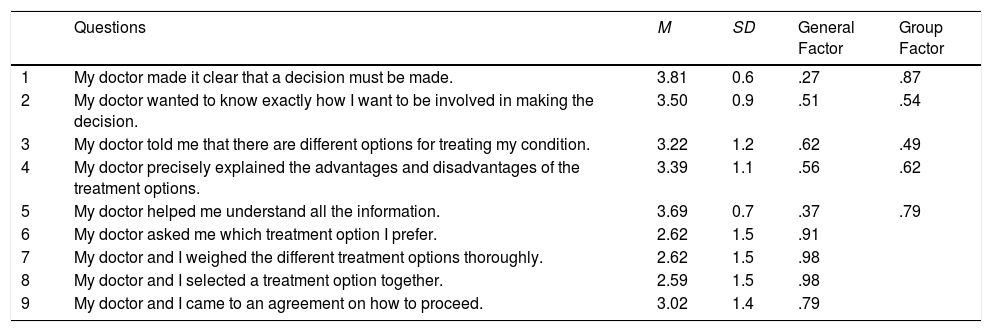
ResultsSMD-Q-9 item descriptive and factor analysisItem means ranged from 2.59 (item 8) to 3.81 (item 1) and the mean sum of SDM-Q-9 was 3.15 (SD=0.9). In general, the item scores were negatively skewed and with high kurtosis values. So, we decided to use the underlying-variables approach, and fit the FA models to the inter-item polychoric correlation matrix (more details in (Ferrando & Lorenzo-Seva, 2013). This approach is quite feasible here given that the test is short and the sample reasonably large. Finally, regarding model adequacy, the KMO index (.87) suggested that the inter-item correlations were substantial and appropriate for being factor analyzed. Table 2 presents the descriptive statistics corresponding to the SDM-Q-9 items.
Descriptive and factor analysis results (bifactor solution) of Shared Decision-Making Questionnaire (SDM-Q-9).
| Questions | M | SD | General Factor | Group Factor | |
|---|---|---|---|---|---|
| 1 | My doctor made it clear that a decision must be made. | 3.81 | 0.6 | .27 | .87 |
| 2 | My doctor wanted to know exactly how I want to be involved in making the decision. | 3.50 | 0.9 | .51 | .54 |
| 3 | My doctor told me that there are different options for treating my condition. | 3.22 | 1.2 | .62 | .49 |
| 4 | My doctor precisely explained the advantages and disadvantages of the treatment options. | 3.39 | 1.1 | .56 | .62 |
| 5 | My doctor helped me understand all the information. | 3.69 | 0.7 | .37 | .79 |
| 6 | My doctor asked me which treatment option I prefer. | 2.62 | 1.5 | .91 | |
| 7 | My doctor and I weighed the different treatment options thoroughly. | 2.62 | 1.5 | .98 | |
| 8 | My doctor and I selected a treatment option together. | 2.59 | 1.5 | .98 | |
| 9 | My doctor and I came to an agreement on how to proceed. | 3.02 | 1.4 | .79 |
Note. M: mean, SD: standard deviation. Score ranges from 0 (strongly disagree) to 5 (strongly agree). Loadings lower than absolute .25 were omitted.
Because a clear final structure was attained in the entire sample, only a summary of the previous exploratory results will be provided here. The unidimensional model with the original 9 items was untenable by all the standards. Omitting item 1 considerably improved the fit bringing it to the lower limits of acceptability and providing an ECV estimate of .80, which means that 80% of the common variance of the item scores can be explained by a general factor.
The solution in two factors had an excellent fit and was interpretable: factor 1 clustered the items 1, 2, 3, 4, and 5 that assess the information and explanations provided to the patient by the physician about treatment and the advantages and disadvantages of the different options. Factor 2 clustered the items 6, 7, 8, and 9 that appraise the choice of the best treatment option for the patient. However, items 1 and 9 were factorially complex, with substantial loadings on both factors. Factor 1 was stronger and better defined, with a replicability H index of .82 (Ferrando & Lorenzo-Seva, 2017) whereas that of factor 2 was only .75. Finally, the estimated inter-factor correlation was rather high: r=.61.
To sum up, the initial analyses suggest that a unidimensional solution omitting item 1 is almost acceptable, whereas the bidimensional solution fits very well and has a clear interpretation, although it consists of two short and highly correlated factors, one of which is relatively weak, with low replicability. Consequently, no highly reliable scores can be expected to be derived from this factor. In view of these results we considered that the most appropriate and parsimonious solution for the SDM-Q-9 items was a bifactor solution (Rodriguez, Reise, & Haviland, 2016) based on all 9 items, with a general factor that describes the entire SDM process, and a group factor defined by items 1 to 5 related to the information and treatment options provided to the patient. The bifactor solution is justifiable, given the data's essential unidimensionality; it also maintains the parsimony and strength advantages of the unidimensional solution (clear interpretation and high reliability of the general factor). At the same time, the additional inter-item covariance between items 1 to 5 that cannot be explained by the general factor is modeled as a group factor, thereby avoiding potential bias on the general factor due to unmodeled inter-item covariance.
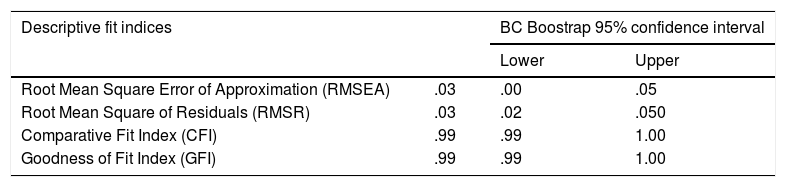
Factor analysis solutionOn the basis of the EFA results summarized above, a bifactor SCFA solution was fitted to the entire sample data with the following specifications: factor 1 (the general factor) was defined by all 9 items, and factor 2 (the group factor) was defined by items 1 to 5. As in the previous EFAs, the bifactor model was fitted by using robust ULS estimation as implemented in FACTOR. Goodness-of-fit results are in Table 3 and indicate an excellent fit.
Robust goodness of fit statistics.
| Descriptive fit indices | BC Boostrap 95% confidence interval | ||
|---|---|---|---|
| Lower | Upper | ||
| Root Mean Square Error of Approximation (RMSEA) | .03 | .00 | .05 |
| Root Mean Square of Residuals (RMSR) | .03 | .02 | .050 |
| Comparative Fit Index (CFI) | .99 | .99 | 1.00 |
| Goodness of Fit Index (GFI) | .99 | .99 | 1.00 |
Note. Cut off criteria: RMSEA ≤.06, CFI and GFI >.95 and RMSR ≤.08.
The general factor in Table 3 is well defined by the 9 items with all loadings >.30 except for item 1 (as expected from previous results). These loadings can be interpreted as item discriminations and are reasonably acceptable for a personality measure. The H-index is therefore rather high: 0.88, meaning that the factor is strong, well defined, and likely to replicate across different samples. The second group factor is mainly defined by items 1 and 5, which have high loadings on it and only moderate loadings on the general. In this case, the H-index is only 0.73, which means that the group factor is far weaker than the general factor, the most common result in bifactor solutions (Rodriguez et al., 2016).
To assess the invariance of the solution described thus far, a series of analyses were performed by splitting the entire sample into subsamples according to gender and pathology type. In all cases, the results were found to be essentially invariant, both in terms of item locations and item discriminations. Thus, there appears to be no differential item functioning for any of the items and the scale is expected to function with the same properties in the general population for which it is intended. Given the space limitations, the invariance results are not provided here, but are available from the authors.
Scoring and reliabilityThe clear bifactor solution discussed above allows two summed scores to be obtained. First is the sum of the scores for all 9 items, which represents the general factor, and so, aims to measure a general dimension of perspectives regarding the decision-making processes. The second score is the sum of items 1 to 5, represents the group factor above, and measures a more specific sub-dimension of information and treatment options provided to the patient. From the H results above and also from basic psychometric principles, the total sum score is expected to be more reliable than the group sum score, and this was indeed the case. The omega reliability estimates were .90 for the total scores and .85 for the group scores. So, both scores achieve a quite acceptable degree of accuracy, and the total scores in particular would be considered as accurate enough for clinical (individual) assessment. Overall, the total scores are more representative of the entire SDM process and will be the ones used in the validity assessments below.
To ascertain whether the reliability of the total scores reflects accuracy at all trait levels, conditional reliabilities were also estimated (see Ferrando & Lorenzo-Seva, 2017). Results revealed that conditional reliabilities were >.85 for a range of trait values between two standard deviations below the mean and two standard deviations above the mean. Hence, not only do the SDM scores possess good overall reliability, but this reliability is also high for almost the entire effective trait range, and the .90 estimate reported above is thus representative of the overall precision of these scores. This result is a positive feature of the instrument and suggests that SDM would enable most respondents to be accurately assessed.
Finally, given the results summarized in this section, and for the benefit of practitioners, a normative table based on the total sum score was constructed based on the entire sample data. The table is provided as supplementary material.
Construct validityConstruct validity was explored by analyzing the product-moment correlations between the total SMD-Q-9 scores (as proxies for the general SDM dimension) and scores from other questionnaires aimed at measuring theoretically-linked dimensions. Results indicated that the total SMD-Q-9 score relates positively with satisfaction regarding the patient-physician relationship (r=.29, p<.001), specifically with the time dedicated (r=.40, p<.001), but not with the information provided (r= -.02, p=.313). Similar results were found with the group factor SDM-Q-9, which related positively with satisfaction regarding the patient-physician relationship (r=.36, p<.001) and time dedicated (r=.32, p<.001), but not with information provided (r=.07, p=.313).
Significant correlations were found between SDM-Q-9, patient version, and SDM-Q-Doc, physician version (r=.14, p<.001), between women (r=.21, p<.001), but not in men (r= -.04, p=.464) and estimated risk of relapse (r=.04, p=.289).
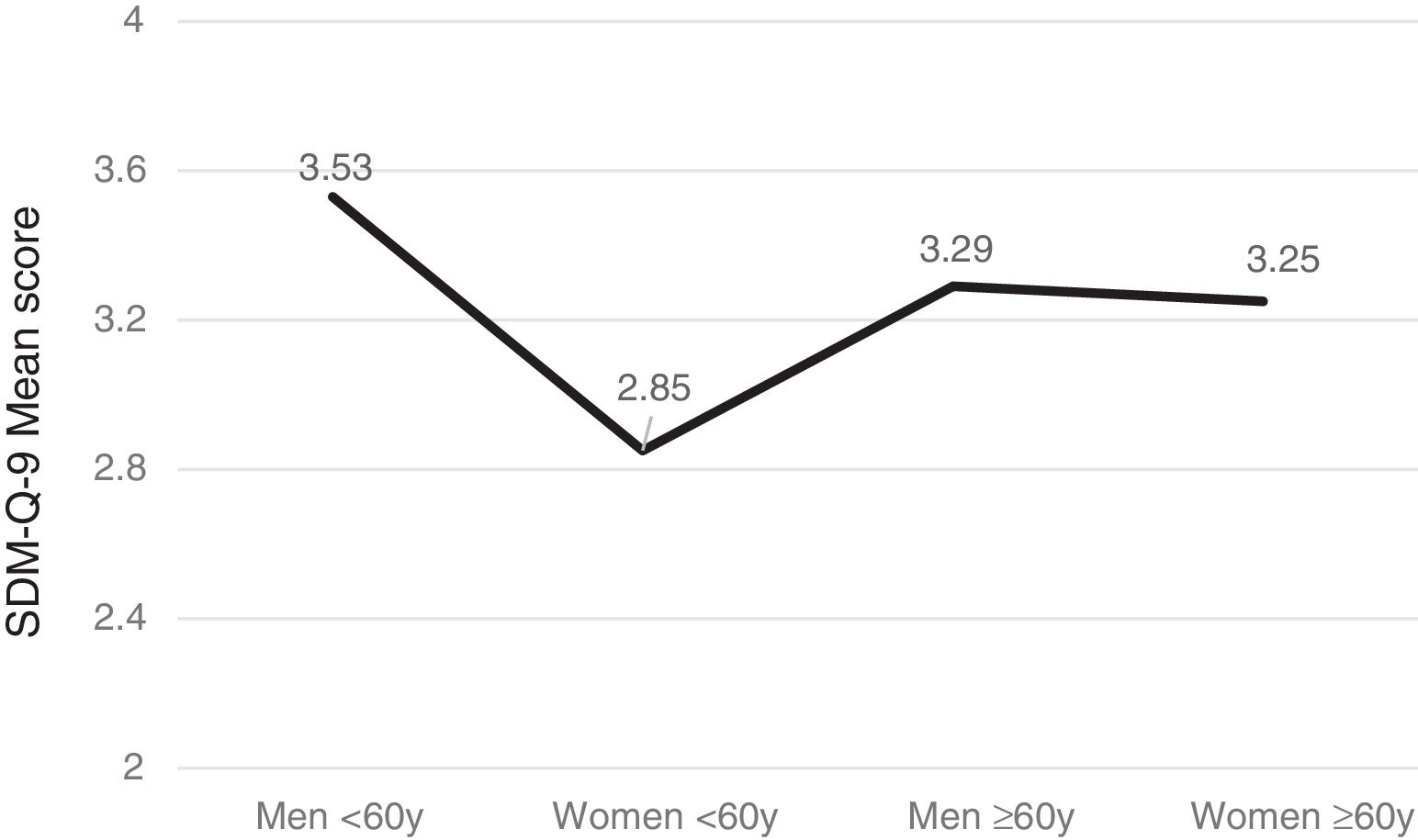
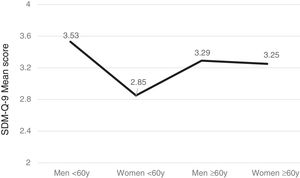
Gender, age and SDM-Q-9Men tended to exhibit greater satisfaction with the SDM than women (F(1,566) =10.96, p<.001) and patients over 60 years (n=288) more than younger ones (n=270) (F(1,556) =5.19, p=.023) with significant intergroup differences (F(3,552)=7.53, p<.001). Furthermore, the subsequent post-hoc Bonferroni analysis uncovered significant gender- and age-based differences (p<.005) such that women under the age of 60 years were the least satisfied group with SDM versus men of the same age, or older women (see Figure 1).
DiscussionThe aim of the present study was to analyze the psychometric properties of SMD-Q-9 created by Kriston et al. (2010) in a population of patients with resected, non-metastatic cancer and in the context of potentially curable disease. To the best of our knowledge, this is the first report of the SMD-Q-9's psychometric properties in oncology, and, in this type of population, it showed several positive properties. First the obtained factorial structure was clear and meaningful, with a strong and replicable general factor and a more specific group factor that can be of interest in finer graded clinical assessment. The present results are compatible with previous results reported by Kriston et al. (2010) and De las Cuevas et al. (2015), in that the scale was considered as essentially unidimensional. In line with these previous studies, we also find item 1 (″My doctor made it clear that a decision must be made″) to be the most problematic, with low discriminating power and high specificity. Second, sum-scores derived from the factor solution, especially those corresponding to the general factor were quite accurate, with an omega estimate .90, in line with previous reported reliabilities.
Validity results were less strong. Convergent validity was explored by comparing it to the SDM-Q-Doc and Satisfaction with the Information (SIS). Only a weak and statistically significant correlation was found between SDM-Q patient version and physician version. Correlations between SDM-Q-9 and SIS were positive and significant, but only insofar as the time dedicated to informing was concerned, but not with the information provided. Thus, the hypothesis of a substantial correlation between the availability of two instruments – the SDM-Q-9 (Kriston et al., 2010) and SDM-Q-Doc (Scholl et al., 2012), comparing the patient's and physician's perspectives on the SDM process – has been slightly established. Patients seem to expect more information from their doctors.
Overall, the psychometric results of our study were consistent with the results from the original German scales, as well as the Dutch and Spanish versions. Differences might be explained by factors such as the scale's ceiling effect, patients’ age and gender, and type of care. As mentioned above, item scores were negatively skewed, which means that the full scale has a ceiling effect. The use of the FA based on polychoric correlations was expected to correct for this problem as far as assessment of factorial structure was concerned. However, the reduced variance due to the end effect can be expected to attenuate both the reliability and the validity estimates based on the sum scores. The ceiling effect might be caused by social desirability (Mead & Bower, 2000) and the patient's wish to please the physician that typically occurs when measuring patient satisfaction (Chewning et al., 2012). Similar results were found by Scholl et al. (2015) who found weak correlations between SDM-Q-9 and OPTION scales. Both instruments assess behavioral aspects of the decision-making process. Moreover, it should be also noted that in our study, the questionnaires were provided after the initial visit to the oncologist and completed immediately afterward. This could increase the social desirability bias and must be taken into account for recruitment in future research.
Our sample consisted of relatively older patients (mean age of 59 years) compared to the samples in the Spanish validation study (mean age, 45 years). Older people are often more satisfied with the information provided by the physician and have less expectations surrounding their participation in SDM (Singh, Butow, Charles, & Tattersall, 2010). Our sample also presents a marginally higher percentage of women (59.5%), similar to the Dutch sample (60%) and slightly lower than the Spanish validation one (70%). Previous studies have found that female cancer patients are more likely to prefer SDM than males (Olson & Windish, 2010; Singh et al., 2010), and demand to participate in the process more than their counterparts.
Some studies suggest that response patterns may differ depending on age, gender, and medical condition (O’Connor et al., 2009). The fact that our sample consisted solely of oncological patients may have contributed significantly to the differences detected. The growing complexity of adjuvant therapies used in the treatment of cancer complicates SDM as it pertains to the best treatment and adds prognostic uncertainty and fear to the negative consequences of inappropriate decisions (Thorne, Oliffe, & Stajduhar, 2016). Future research concerning the construct validation and predictive validity of the scale are needed, including different subtypes of cancer and at different stages.
Finally, the SDM-Q-9 may not capture aspects of the visit, such as communication style, body language, or empathy, all of which correlate highly with satisfaction.
This study presents certain limitations that must be taken into account for future research. First of all, although our sample size is large, participants were patients with a localized tumor who had undergone surgery and were candidates for adjuvant chemotherapy. In the future, we would advise expanding the sample to include other tumor stages and types with the aim of confirming these results, as well as to compare different clinical-pathological and social variables. Secondly, the SDM-Q-9 self-report subjective measures may not accurately reflect patients’ experiences, expectations, and behavior, having limitations such as response bias (social desirability, inaccurate memory, etc.) and their difficulty in fully comprehending the SDM process (Shay & Lafata, 2015). Finally, in addition to this design, it would be fitting to explore the dynamic nature of SDM processes with other longitudinal studies that enable SDM to be evaluated more comprehensively, exploring its effects before and after a decision is made.
In conclusion, the “Shared Decision Making Questionnaire” applied to patients with cancer possesses adequate psychometric properties, similar to those obtained by Kriston et al. (2010), Simon et al. (2006), and De las Cuevas et al. (2015). The results of this study prove that it is a valid and reliable tool for analyzing and attaining greater insight into the SDM process. On the other hand, knowing which conditions help or hinder engagement in this decision-making process can help to establish the clinical conditions necessary to enhance patients’ wellbeing.
SDM is a process aimed at learning patients’ preferences and needs and toward empowering them to take an active role in caring for their health in line with their wishes. The SDM-Q-9 can be useful to analyze these patients’ perspective of the SDM and as an indicator of quality and satisfaction with healthcare services.
Funding and acknowledgementsThis work was funded by the Spanish Society of Medical Oncology (SEOM) in 2015. The sponsor of this research has not participated in data collection, analysis, or interpretation, in writing the report, or in the decision to submit the article for publication. The authors would like to thank the investigators of the Neocoping study (coping, shared decision-making and quality of life in patients with early stage cancer treated with adjuvant chemotherapy) and the Supportive Care Working Group of the Spanish Society of Medical Oncology (SEOM).
Sources of fundingThis work was funded by the Spanish Society of Medical Oncology (SEOM) in 2015. The sponsor of this research has not participated in data collection, analysis, or interpretation, in writing the report, or in the decision to submit the article for publication.
The authors would like to thank the investigators of the Neocoping study (coping, shared decision-making and quality of life in patients with early stage cancer treated with adjuvant chemotherapy) and the Supportive Care Working Group of the Spanish Society of Medical Oncology (SEOM).