Inhibition is crucial for controlling behavior and is impaired in various psychopathologies. Neurofeedback holds promise in addressing cognitive deficits, and experimental research is essential for identifying its functional benefits. This study aimed to investigate whether boosting sensorimotor activity (SMR) improves inhibitory control in a final sample of healthy individuals (N = 53), while exploring the underlying neurophysiological mechanism.
MethodParticipants were randomly divided into two groups: one receiving SMR neurofeedback training to enhance sensorimotor activity within the 12–15 Hz frequency range, and the other receiving sham feedback. Inhibition performance and neural correlates were evaluated with a Go-NoGo task before (T0) and after (T1) 10 neurofeedback sessions using event-related potentials. Data were analyzed via ANOVAs and regression analyses.
ResultsCompared to placebo, the active group demonstrated higher absolute SMR power (p = 0.040) and improvements in inhibitory control, including faster response times and fewer inhibition errors (p < 0.001, d = 0.76), associated with a larger NoGoP3d amplitude (p < 0.001, d = 0.69). A positive correlation between the increase in SMR power and the rise in NoGoP3d amplitude (β=0.46, p = 0.015) explains 21 % of the observed variance.
ConclusionsUptraining SMR power is linked to heightened utilization of neural resources for executing optimal inhibition responses. These results uphold its effectiveness in cognitive rehabilitation.
Interventions within psychiatric and neurological clinical settings have progressed towards integrating brain computer interfaces (BCI), i.e. tools allowing a communication link between the brain and an external device, alongside conventional methods like psychotherapy and medication (Pindi et al., 2022; Saha et al., 2021). Among these innovative interventions, neurofeedback, developed in the 1960s, aims at reconditioning brain activity patterns that are correlated with deviant psychological and behavioral processes (Mahrooz et al., 2023). In essence, neurofeedback utilizes specialized neuro-imaging instruments to mirror physiological processes that individuals are typically not consciously aware of. During a neurofeedback session, participants are provided with immediate feedback on particular brain activities. Upon successfully generating the desired patterns, individuals are met with positive reinforcement, fostering an associative learning process that encourages the brain to produce more of these beneficial patterns. In this way, patients dynamically modulate their cerebral state with the long-term goal of attaining a more balanced and optimal state of brain function (Arns et al., 2020; Viviani & Vallesi, 2021).
A thoroughly investigated EEG neurofeedback approach, identified as a 'standard neurofeedback protocol,' is the sensori-motor rhythm (SMR) method, which centers on acquiring voluntary control over SMR activity (Arns et al., 2020). The sensori-motor rhythm constitutes a singular type of oscillatory activity within the 12 to 15 Hz frequency range, occurring over the sensori-motor strip and associated with a relaxed state and focused mind (Micoulaud-Franchi et al., 2019). Significant scientific advancements have substantially improved our understanding of the neurophysiological mechanisms that govern the regulation of SMR activity and its contemporary implications for cognitive functions (Kober et al., 2015; Ribeiro et al., 2023). Associated with a state of relaxed alertness, this distinct brain activity is linked to a reduced somatosensory information flow (Micoulaud-Franchi et al., 2019), giving rise to what Sterman referred to as “internal thalamic inhibition” (Sterman & Bowersox, 1981; Sterman, 1996). Under current theoretical paradigms, this process liberates neural resources that can be subsequently allocated for use by alternative networks (Kober et al., 2015; Micoulaud-Franchi et al., 2019; Sterman, 2000), potentially enhancing diverse cognitive functions (Egner & Gruzelier, 2004; Sterman, 1996, 2000). Empirical evidence supports the positive correlation between uptraining SMR activity and cognitive improvements (Gruzelier, 2014; Kober et al., 2015), making this protocol valuable for alleviating symptomatology associated with neurological, motor, or psychiatric disorders (see Marzbani et al., 2016 and Ribeiro et al., 2023 for meta-analyses).
Despite advancements in comprehending SMR neurofeedback mechanisms and encouraging research outcomes, a substantial heterogeneity remains in reported results across the scientific literature (Dousset et al., 2020). Experts attribute the lack of robust evidence to inconsistencies in study designs and objective outcome evaluations (Arns et al., 2017; Ribeiro et al., 2023; Ros et al., 2020). This discrepancy precludes the definitive identification of clinical benefits associated with SMR neurofeedback training as a form of clinical intervention for any pathology. In response, researchers have introduced the CRED-nf checklist, derived from consensus, to elevate standards in reporting experimental design within the field (Ros et al., 2020). In alignment with this scientific community's initiative, the present study aims to comprehensively evaluate the impact of 10 sessions of neurofeedback training, specifically targeting an increase in sensori-motor activity (12–15 Hz), on cognitive inhibitory control by using a placebo-controlled design, combined with the utilization of event-related potentials (ERPs) as indicators of brain changes, and the establishment of a connection between behavioral and neurophysiological levels within the context of inhibitory control. Indeed, response inhibition is a central component of executive control, a higher-order cognitive process that serves future-oriented goals and that is central to current theories of various psychopathologies characterized by poorly regulated and impulsive behaviors (Abramovitch et al., 2021). Moreover, ERPs provide a dependable reflection of cognitive-related cerebral dynamics, offering the unique capability of monitoring cognitive-processing streams and their evolution with high temporal resolution (Campanella, 2023).
The primary aim of this study is to empirically examine the hypothesis that augmenting sensori-motor rhythm activity in healthy individuals results in enhanced inhibitory control, defined by the parameters of a fast-paced Go-NoGo task as a reduced occurrence of commission errors (i.e. hits on NoGo trials) conceivably associated with faster responses to Go trials (Wessel, 2018). A secondary objective is to elucidate the neurophysiological mechanism underlying this phenomenon, with the goal of validating the theory that an increase in SMR power leads to the release of neural resources available for utilization by other networks. In this context, ERPs were recorded before (T0) and after (T1) training sessions to capture specific and well-known inhibitory brain markers (N2/P3 components), which have already been modulated by cognitive and/or neuromodulatory training programs in healthy participants (Dousset et al., 2021). Our main hypotheses posit that, compared to participants receiving a placebo, those engaged in active neurofeedback (NF) will exhibit, across the 10 training sessions, an increase in SMR power correlated with heightened inhibitory performance and increased neural activities. Statistically, we will initially verify the effectiveness of neurofeedback training, examine the progression of inhibition capacities at both behavioral and neurophysiological levels, and subsequently evaluate the correlation between the two (Weber et al., 2020).
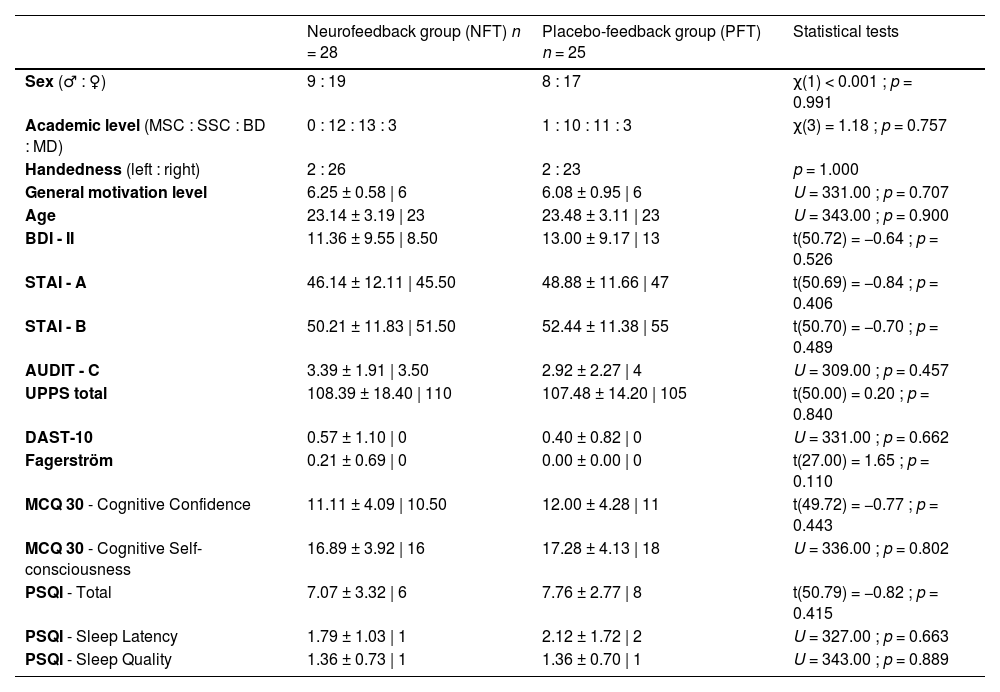
Materials and methodsParticipants and ethic statementHealthy young participants (between 18 and 30 years-old) were recruited through social media platforms to take part in the study between January 2021 and March 2023. Before their involvement, the participants completed several questionnaires to assess various psychological measures and an anamnesis, ensuring they were not on any medications and had no history of neurological or psychiatric disorders. Additionally, participants were excluded if they engaged in heavy social drinking (over 14 units of alcohol weekly) or used cannabis, as these substances can influence inhibition performance as well as ERP components (Marvi et al., 2023). The study enrolled a total of 75 participants who were randomly assigned to: an (1) active neurofeedback group (NFT) aiming at enhancing the sensori-motor rhythm (12–15 Hz) and a (2) placebo-feedback group (PFT) in which participants enhanced random frequency bands. Out of the initial N = 75 participants recruited, attrition and exclusion criteria resulted in 53 participants with analyzable data for the final sample. Details of the group's characteristics are provided in Table 1 (please refer to supplementary materials for detailed a priori power calculation and questionnaires).
Abbreviations: MSC (Middle School Certificate), SSC (Secondary School Certificate), BD (Bachelor's Degree), MD (Master's Degree), BDI (Beck Depression Inventory), STAI (State and Trait Anxiety Inventory), AUDIT (Alcohol Use Disorder Test), DAST (Drug Abuse Screening Test), MCQ (Metacognition Questionnaire), PSQI (Pittsburgh Sleep Quality Index). The term ‘sex’ refers to the biological and physiological characterisctics of individuals. Statistical analyses included the Chi-square test, Fisher's exact test, Welch test, and Mann-Whitney test. Descriptive data are presented as numbers, or mean ± SD | median.
The research received approval from the local ethics committee at Brugmann Hospital (Hospital Ethics Committee OM026 2019/51 dated April 9, 2019). All participants gave informed written consent after receiving comprehensive information about the study, adhering to the principles stated in the Declaration of Helsinki. We adhered to the CONSORT checklist when writing our report (Schulz et al., 2010). It is important to note that participants did not receive any financial compensation (refer to supplementary materials for details). Recruitment ended in 2023 upon achieving the predetermined sample size through a priori power calculation. This study served as a pilot to assess the improvement of cognitive function using SMR neurofeedback training for the clinical trial registered with the identifier NCT05913518 on ClinicalTrials.gov. This clinical trial aims to investigate neurofeedback use in relapse prevention among patients diagnosed with alcohol dependency undergoing an Alcohol Detoxification Program at Brugmann Hospital (Brussels, Belgium).
EquipmentEEG and the Go-NoGo taskElectroencephalograms (EEGs) were recorded with 32 electrodes mounted in an electrode Quick-Cap. The electrode positions included the standard 10–20 system locations. The recordings were made with a linked mastoid physical reference (M1, M2). The EEGs were amplified with battery-operated ANT amplifiers using a gain of 30 000 and a band-pass filter of 0.01 to 100 Hz. The impedance of the electrodes was maintained under 10 kOhms. The EEGs were recorded at a sampling rate of 1024 Hz (ANT Eeprobe software®) during a Go-NoGo task (please refer to supplementary materials for detailed procedure of the Go-NoGo task).
NeurofeedbackNeurofeedback training was administered using the THERA PRAX NeuroConn GmbH system (version 2.6.13). The recordings were obtained using Ag/AgCl electrodes, with the ground electrode positioned on the left mastoid and the reference electrode on the right mastoid. The primary electrode was placed at C3. Additionally, four electrodes were used to monitor eye movements: VEOG I and II were placed above and below the left eye to control for vertical movements, and HEOG I and II were placed on the left and right temples to control for horizontal movements. The signal was amplified using DC-EEG amplifier. Detailed neurofeedback and placebo-feedback protocols are described in the supplementary materials, under section I.3 Neurofeedback and placebo-feedback protocols.
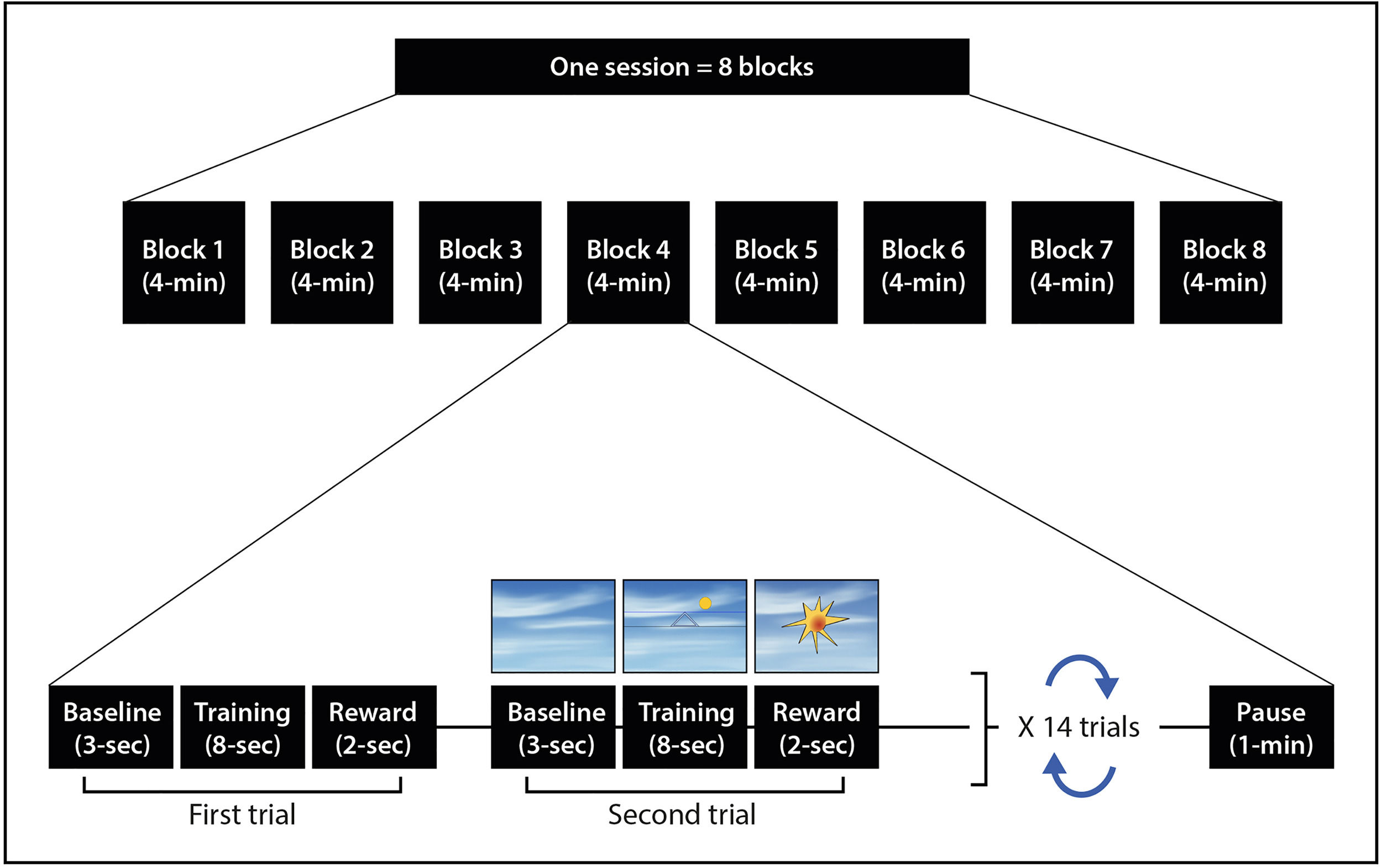
ProcedureWe intended to provide 10 repeated neurofeedback training sessions. Each session followed a standardized procedure and lasted for 32 min. In every session, participants completed eight 3-minute blocks of instrumental conditioning, with each block consisting of 14 consecutive trials (see Fig. 1). The NFT group focused on increasing activity in the 12–15 Hz SMR range, while the PFT group targeted random frequency ranges between 7 and 20 Hz, excluding the 12–15 Hz SMR range. The protocol was based on the approach used by Hoedlmoser et al. (2008) and Schabus et al. (2014, 2017). To assess the neurocognitive impact of this neurofeedback training procedure on inhibitory control, participants completed a Go-NoGo task while EEG recording was performed. This task was administered both immediately before the first neurofeedback session and immediately after the final neurofeedback training session. The procedure has been done in a double-blind fashion. Both the experimenter and the participant were blinded to the condition using an NFT/PFT code list. This list employed letters from A to T to represent each targeted frequency in every session. Importantly, for each participant, the order of NF training frequency code list was randomly generated with the sample() function on RStudio 1.4.1103.
Data preprocessing and statistical analysesMeans and standard deviations were used to measure central tendency and dispersion. Repeated measures ANOVAs were employed to assess both within-subject and between-subject comparisons. Paired comparisons were conducted using Student's t-tests, while Welch test was preferred for between-subject comparisons (Delacre et al., 2017). Equivalent non-parametric tests were used when the normality assumption was not met.
Neurofeedback data analysisFor neurofeedback data analysis, we employed Python version 3.11 along with the MNE-Python library (Gramfort et al., 2013). During training, artifacts with an amplitude exceeding 200 microvolts and eye movements were corrected automatically online by the internal THERA PRAX algorithm (please refer to supplementary materials for details). Subsequently, we decided to exclude remaining EEG artifacts using a mathematical approach; specifically, artifacts with an amplitude equal to or exceeding 100 microvolts were rejected. In contrast to the prevalent practice of employing subjective visual inspection, as commonly observed in most EEG-based experimental studies, the mathematical nature of our method supports scientific replicability.
To preprocess the data, a 4th-order Butterworth filter was applied to obtain the distinct frequency ranges: theta (6–8 Hz), alpha (8–11 Hz), SMR (12–15 Hz), and beta (15–20 Hz). We used a sliding window with epochs of 1 second and a shift of 1/8 second. For each frequency band, absolute power was quantified by calculating the average of squared values [mean(values2)] expressed in μV2. Afterward, for the sake of interpretability, a logarithmic transformation [log(1 + mean(values2))] was applied to address skewness. The complete neurofeedback data processing script can be found in the GitHub repository: https://github.com/tmonseigne/SMR-Neurofeedback-Analysis.
Statistically, a manipulation check has been performed to assess the efficacy of neurofeedback training at the group level, in order to verify whether there has been a change between the active neurofeedback group and the placebo-feedback group in the absolute power of SMR (12–15 Hz) as well as in the other frequency bands: theta (6–8 Hz), alpha (8–11 Hz), and beta (15–20 Hz) (Alkoby et al., 2018). Considering the notable impact of factors like motivation or fatigue on neurofeedback performance (Kadosh & Staunton, 2019) and the assumption of a non-linear learning curve, we contend that an accurate assessment involves considering the complete training - spanning from session 1 to session 10 in this case – using the area under the curve method (AUC) to encompass the entirety of performance, including both less optimal and more successful sessions (please refer to supplementary materials for a details). For each frequency band, absolute power was calculated for each session. As a reference, the absolute power value of the initial session was subtracted from the absolute power value of each subsequent session. Afterward, the areas under the curves were computed, aggregated, and subjected to comparison via the Welch test (Delacre et al., 2017), or the corresponding non-parametric Mann-Whitney U test if normality assumption was not met, considering the Group as the between-subjects factor.
EEG data analysisOnce acquired, a band-pass filter 0.3 to 30 Hz was applied and response-locked epochs of 800 ms (200 ms before and 600 ms after the stimulus onset) were created (ANT Eeprobe software®). A cutoff of 3 standard deviations around the mean was used to define trials that were contaminated either by eye movements or muscular artifacts, which were detected offline and discarded from further analyses to only analyze the artifact-free trials. The "difference" wave, obtained by subtracting Go trials from NoGo trials, is traditionally considered to reflect the inhibitory Go/NoGo effect itself, as indicated by N2d and P3d components (Huster et al., 2013). For each participant, ERP waves were separately calculated for Go and No-go stimuli on each electrode. The difference in NoGo N2 and P3 amplitude (NoGoN2d and P3d; NoGo minus Go wave) was determined as the peak of negative and positive values within the 150–300 msec and 300–500 msec intervals after stimulus onset, respectively. This analysis was conducted in a standard cluster of frontocentral electrodes (FC1, FC2, Cz, and Fz) (Luck, 2014). Incorrect responses (miss for Go, false alarm for NoGo) were excluded from the EEG analyses.
Statistically, a total of six omnibus repeated measures ANOVAs were conducted, taking into account the effects of Group (with two levels: active neurofeedback group and placebo-feedback group) as a between-subjects factor, and Session (with two levels: T0 and T1) as a within-subjects factor, on both behavioral measures (i.e. the number of commission and reaction times) and electrophysiological measures (including the amplitudes and latencies of NoGoN2d and NoGoP3d components). To further explore significant main effects and interactions, post-hoc analyses were conducted. Importantly, as highlighted in the literature (Armstrong, 2014; Barnett et al., 2022; Rubin, 2021), prior applications of corrections to address challenges associated with multiple testing have frequently been misapplied. Streiner and Norman (2011) emphasize that the choice to implement a correction depends on specific circumstances and must be justified. Considering our study's foundation in established theory and its focus on alleviating cognitive deficits, our choice of Bonferroni-Holm's correction method aims to ensure high certainty in treatment comparisons, by prioritizing conclusive findings over hypothesis generation (Streiner & Norman, 2011), while being less conservative than the standard Bonferroni correction. This decision is made to manage the family-wise error rate and reduce type I error risk, even at the potential expense of increasing type II errors.
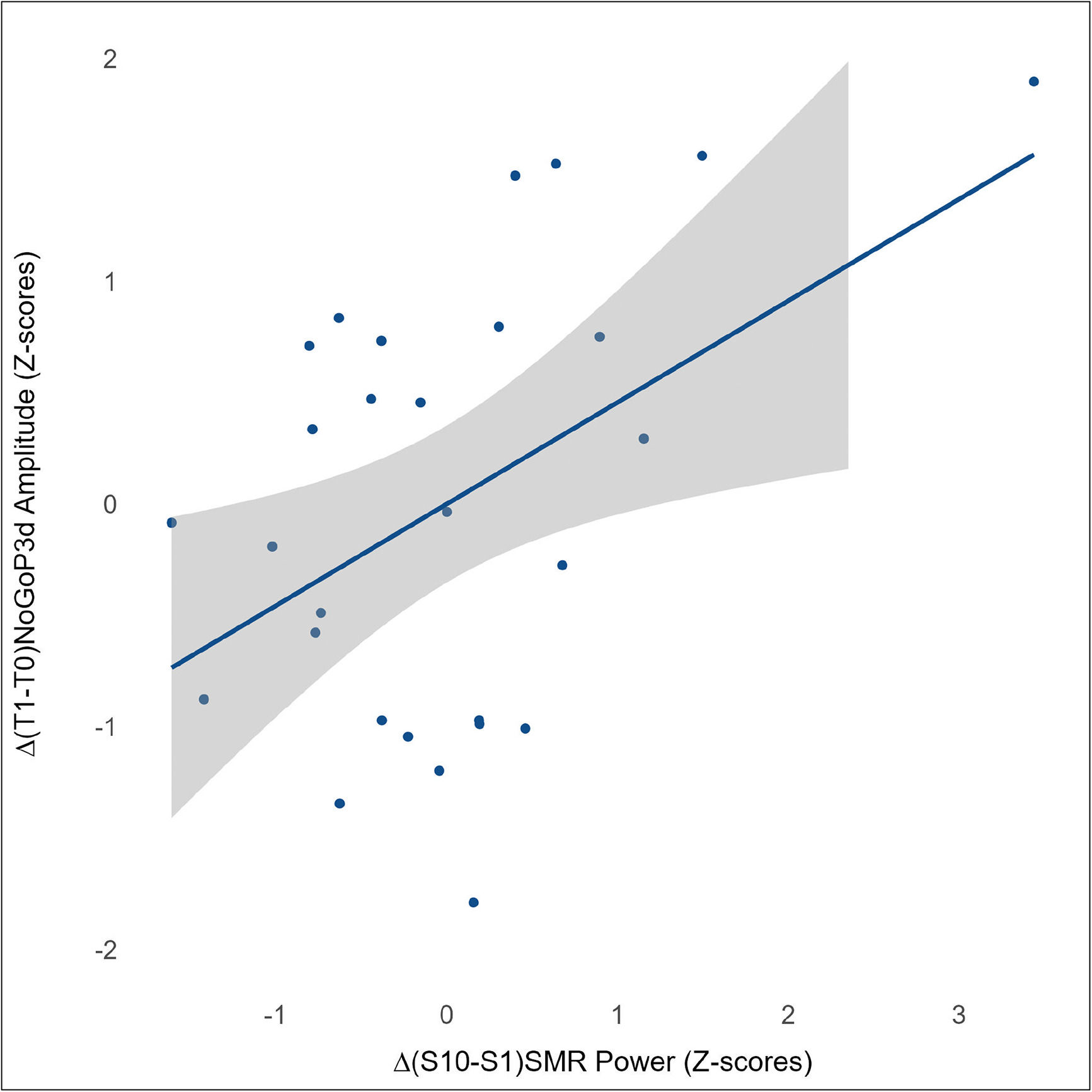
Afterward, under the assumption of a linear connection between the changes in SMR's power activity between session 1 and session 10 (independent variable), and the rise in the amplitude of the ERP components from T0 to T1 (dependent variable), we employed a linear regression to both model and quantify the direction of this influence in the active neurofeedback group, utilizing z-scores: ∆NoGoP3d amplitude = β0 + β1.∆SMR_power + ε.
ResultsDescriptive statisticsThe statistical analyses conducted on demographic and psychological variables are detailed in Table 1. No significant differences were observed between the NFT and PFT groups (p's > .05).
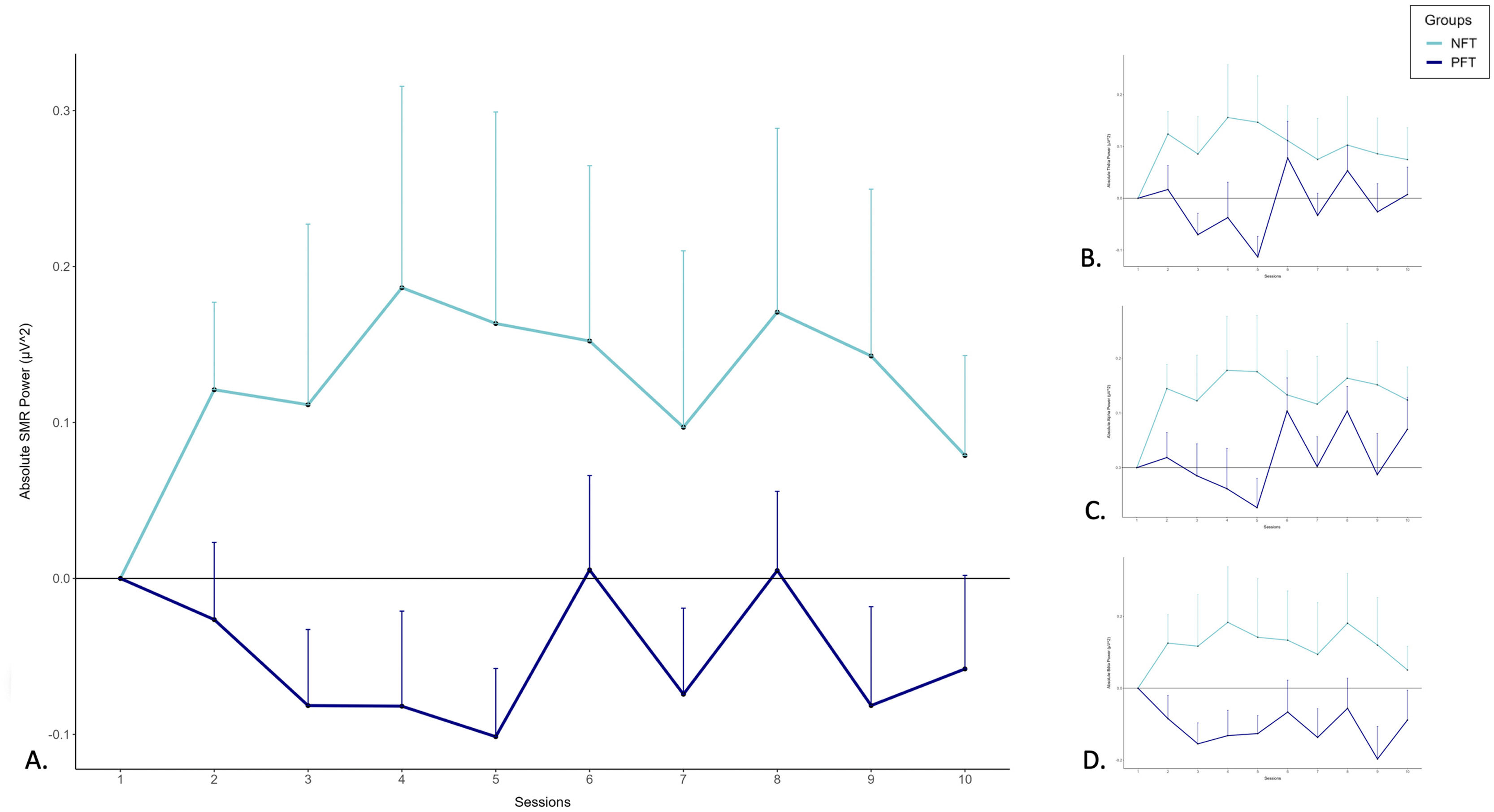
Neurofeedback dataThe results of the non-parametric Mann-Withney U test conducted on the AUC of centered absolute SMR power values (μV2) revealed a significant difference (U = 235.00, p = 0.040) between the NFT group (1.17 ± 4.22) and the PFT group (−0.44 ± 1.75) (see Fig. 2). No significant differences were found for centered absolute power values of Theta (U = 253.00, p = 0.084), Alpha (U = 259.00, p = 0.105), and Beta (U = 257.00, p = 0.098).
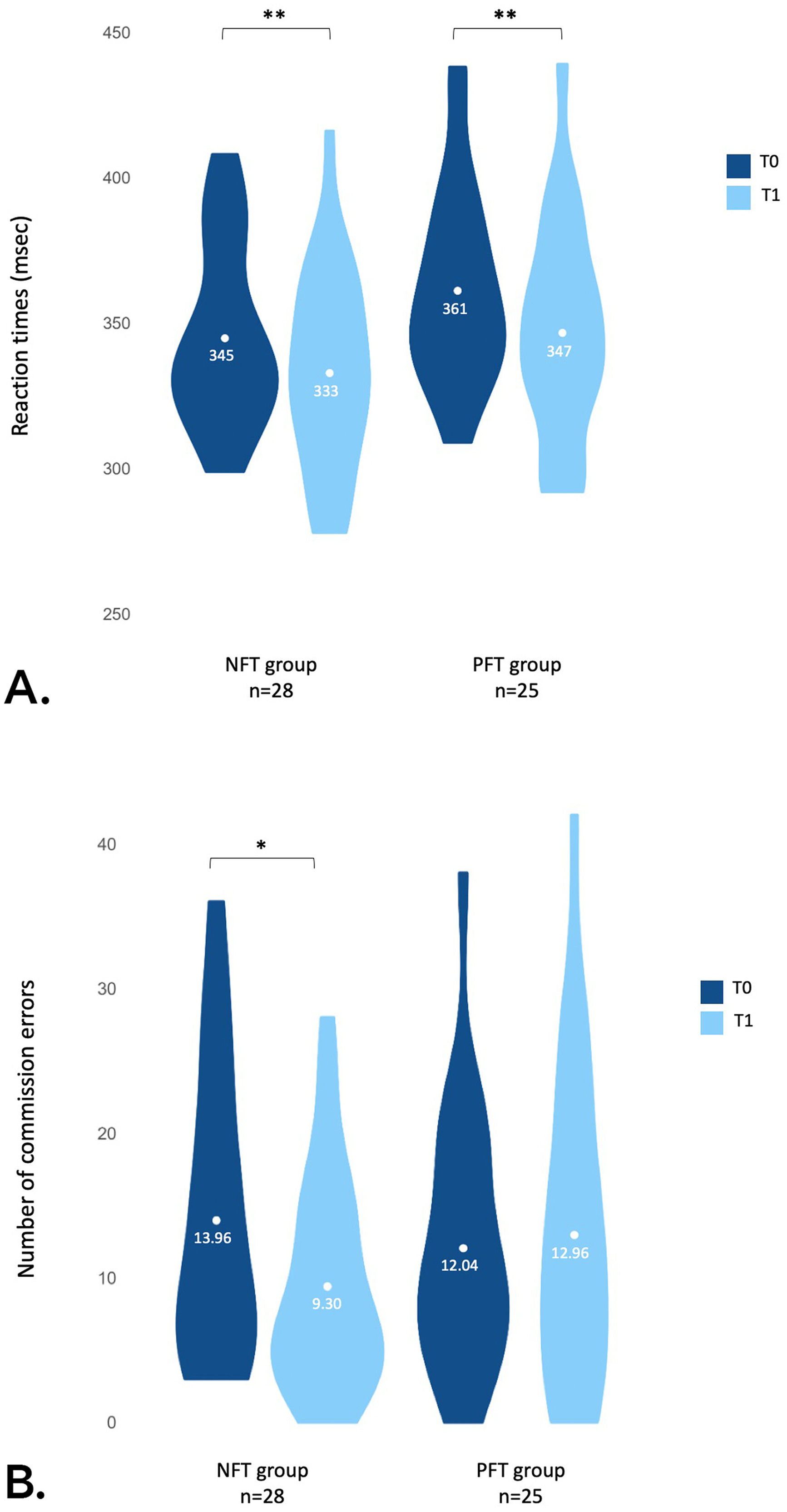
Go/NoGo task - Behavioral dataThe results of the 2 × 2 ANOVA conducted on the reaction times (msec) revealed a significant main effect of session [F(1, 51) = 8.60, p = 0.005, η2p = 0.14] characterized by a decrease between T0 (352.49 ± 32.40) and T1 (339.34 ± 35.28), all groups combined (see Fig. 3.A).
The results of the 2 × 2 ANOVA conducted on the number of commission errors revealed a significant interaction between Group and Session [F(1, 51) = 4.30, p = 0.043, η2p = 0.08]. In the active neurofeedback group, the post-hoc analysis revealed a significant decrease (t(27) = 3.99, p < 0.001, d = 0.76) in the number of commission errors between T0 (13.96 ± 9.84) and T1 (9.39 ± 7.18). No significant differences were found between T0 and T1 in the placebo-feedback group (PFT) (t(24) = −0.37, p = 0.358) (see Fig. 3.B).
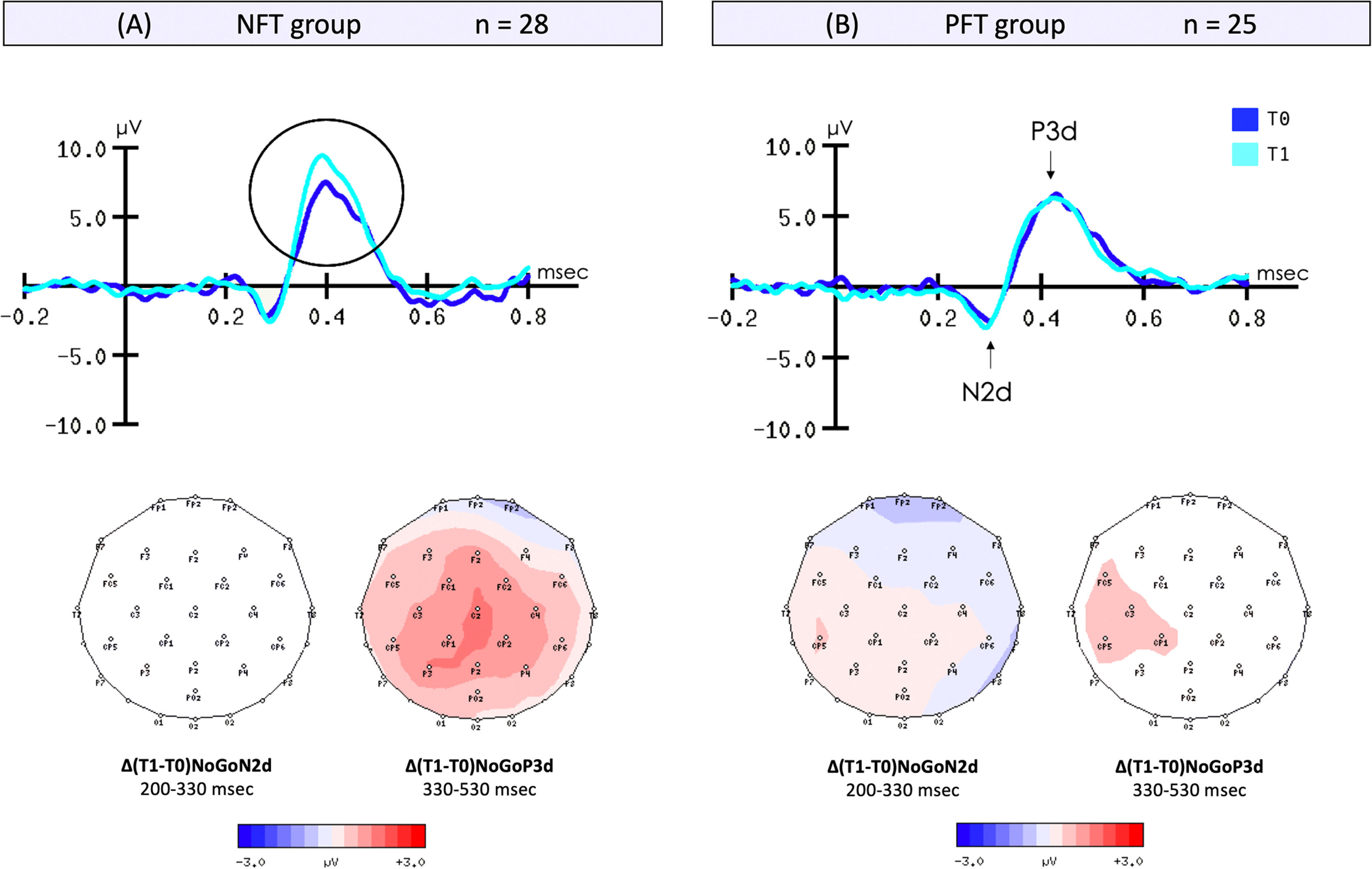
Go/NoGo task - Neurophysiological dataThe results of the 2 × 2 ANOVA conducted on the amplitude of the NoGoP3d component (μV) revealed a significant Group*Session interaction [F(1, 51) = 5.49, p = 0.023, η2p = 0.10]. In the active neurofeedback group, the post-hoc analysis uncovered a significantly greater amplitude of the NoGoP3d component (t(27) = -3.64, p < .001, d = 0.69) at T1 (11.36 ± 4.58) compared to T0 (9.06 ± 3.23). No significant differences were found between T0 and T1 in the placebo-feedback group (t(24) = 0.33, p = 0.374) (see Fig. 4).
Grand-averaged event-related potential (ERP) waveforms for NoGoN2d and NoGoP3d obtained at the Fz electrode (selected for optimal signal quality) during T0 and T1 for: (A) the NFT group and (B) the PFT group; and scalp topographies of the difference ∆(T1-T0) for NoGoN2d and NoGoP3d.
The results of the 2 × 2 ANOVA conducted on the latency of the NoGoP3d component (msec) revealed a significant main effect of group [F(1,51)=12.70, p < .001, η2p = 0.20] characterized by a shorter latency in the active neurofeedback group (NFT) (397.96 ± 6.57) compared to the placebo-feedback group (426.38 ± 3.86), all sessions combined.
The results of the 2 × 2 ANOVA conducted on the amplitude of the NoGoN2d component (μV) showed no significant effects (p's > .05).
The results of the 2 × 2 ANOVA conducted on the latency of the NoGoN2d component (msec) revealed a significant main effect of group [F(1,51)=5.44, p=0.024, η2p = 0.10], characterized by a shorter latency in the active neurofeedback group (NFT) (279.75 ± 1.62) compared to the placebo-feedback group (PFT) (295.15 ± 7.11), all sessions combined.
For information on the correlational analysis between behavioral and neurophysiological measures, please refer to supplementary materials (section II.4).
Regression analysisA simple linear regression analysis was performed to evaluate the presumed association between the augmentation of the SMR rhythm's power (μV2) and the rise in the amplitude of the NoGoP3d component (μV) between T0 and T1 within the active neurofeedback group (NFT), by using z-scores. The regression model demonstrated statistical significance [F(1, 27)=6.84; β = 0.46; p = 0.015], explaining 21 % of the variance in the amplitude of the ∆(T1-T0)NoGoP3d component (R2 = 0.21) (see Fig. 5). Please refer to supplementary materials for residuals and QQ plots.
DiscussionThe objective of this study was to empirically examine whether the uptraining of sensori-motor rhythm (SMR) activity in healthy individuals could improve inhibitory control by retrieving neural resources. Accordingly, the study sought to elucidate the related neurophysiological mechanisms through the analysis of event-related potentials, aiming to validate the theory that an increase in SMR power facilitates the release of neural resources for utilization by other networks.
Firstly, our findings indicate a significant difference between the placebo-feedback group and the active neurofeedback group regarding the efficacy of the neurofeedback training. Specifically, the NFT group exhibited a significantly higher power within the SMR 12–15 Hz frequency band, when compared to the PFT group. As a requisite condition, this observation underscores the NFT group's ability to substantially augment SMR power during training, all sessions comprised. Considering the notable impact of factors like motivation or fatigue on neurofeedback performance, the AUC technique was chosen over the commonly used regression slope analysis as we do not assume the learning curve to be linear (please refer to supplementary materials for a detailed discussion).
Secondly, regarding functional outcomes at the behavioral level, our findings highlight a notable improvement in the performance of the NFT group compared to the PFT group in the Go-NoGo task. Both groups exhibited a significant reduction in reaction times between T0 and T1; however, uniquely, the NFT group demonstrated a concurrent and significant decrease in the number of commission errors (i.e. hits on NoGo trials) with a large effect size as per Cohen's convention. Hence, participants in the NFT group not only showed increased speed but also manifested a reduced occurrence of inhibition errors. While a significant positive linear association between improvement in commission errors on the Go-NoGo task and changes in NoGoP3d amplitude was not observed—likely due to insufficient statistical power—the literature strongly suggests that changes in behavioral performance is closely associated with neurophysiological changes detected by ERPs (Pires et al., 2014). Indeed, serving as a dependable source of information regarding cerebral dynamics related to cognition, ERPs enable the monitoring of cognitive-processing streams with high temporal resolution (Campanella, 2023). In the context of the Go-NoGo task, the N2/P3 complex serves as a reliable indicator of the neural processes associated with inhibitory control (Luck, 2014): with the NoGoN2d component reflecting the initial midfrontal response related to conflict monitoring and signaling the need for inhibition (Huster et al., 2013), and a later fronto-central response, the NoGoP3d, reflecting more elaborative processing such as motor inhibition (Enriquez-Geppert et al., 2010). In our statistical analyses, we primarily attribute the main differences in N2/P3 latencies between groups to sample biases. While the PFT group exhibited no discernible alteration in the amplitude of the N2/P3 complex, the NFT group manifested a remarkable and statistically significant increase in the amplitude of NoGoP3d component between T0 and T1, with an effect size ranging between medium and large as per Cohen's conventions. Hence, EEG data imply that, in the NFT group, the process of inhibition was engaged to a different degree across sessions with an increased intensity of neural response at T1, indicative of a greater deployment of neural resources during task performance (Handy, 2013) and suggestive of a more optimal functioning. Also, as evidenced by the outcomes of the linear regression analysis, a substantial positive correlation is observed between the augmentation in absolute SMR power and the corresponding increase in NoGoP3d amplitude from T0 to T1, in the NFT group. Notably, 21 % of the variance in NoGoP3d amplitude can be attributed to the increase in absolute SMR power.
These findings align with the theory positing the impact of SMR neurofeedback training on cognitive abilities (Kober et al., 2015; Micoulaud-Franchi et al., 2019). During conditioned SMR production, the interplay between neuronal populations in ventro-basal nuclei of the thalamus, thalamic reticular nucleus, and sensori-motor cortex results in “internal thalamic inhibition” with suppression of somatosensory information passage to the cortex (Kober et al., 2015; Sterman, 2000). Also, the trained SMR response is linked to the reorganization of the neuronal function within the sensorimotor system, involving a specific reduction in cellular activity and reflex excitability in the motor pathway, resulting in reduction of motor interference (Micoulaud-Franchi et al., 2019; Sterman, 2010). Although appropriate neuroimaging techniques are required to verify the following assumption, our results align with previous findings by suggesting the hypothesis that the NFT group, by uptraining the power of their SMR activity, might have enhanced internal thalamic inhibition that would have resulted in a reduced processing of somatosensory information and attenuated motor excitability. Hypothetically, the newly available neural resources could then be efficiently used to support the execution of an optimal inhibitory response by key regions, including the supplementary motor area (SMA) and pre/SMA located in the sensorimotor cortex, along with other regions of the frontal network such as the inferior prefrontal gyrus (Swick et al., 2011; Wolpe et al., 2022). Integrating the fMRI imaging technique may provide more tangible insights into the underlying functional mechanisms.
Overall, since changes in SMR power during neurofeedback training influence brain dynamics as assessed through ERPs in the Go-NoGo task, our results attest the ability of neurofeedback to reorganize neural networks, making neurofeedback a prospective tool for achieving sustained recovery in neuropsychiatric illnesses (Dehghani et al., 2023).
One noteworthy finding in the current investigation relates to the fact that only the NoGoP3d component exhibited an increase in amplitude, while the NoGoN2d amplitude remained unaltered in the NFT group. The neurophysiological data here suggest a narrowed influence of neurofeedback training as only one of the two subprocesses associated with response inhibition appeared to be influenced by SMR neurofeedback training. Interestingly, this observation is consistent with the results reported by Bluschke et al. in 2016, revealing a distinct influence of 16 sessions of Theta/beta-ratio neurofeedback training exclusively on the amplitude of the NoGoP3 component among a group of children with ADHD (Bluschke et al., 2016). A conceivable explanation is that both Bluschke et al. (2016) and our study employed a fast-paced Go-NoGo task to assess inhibition. This means participants were instructed not only to press on Go trials and withhold from pressing on NoGo trials but also to respond as quickly as possible on Go trials (Wessel, 2018). Consequently, this introduced an additional factor –urgency and time constraints– that accentuated the motor aspect of inhibitory response, represented by the NoGoP3 component. In a future study, it would be worthwhile to investigate whether there is an increase in both NoGoN2 and NoGoP3 amplitudes when employing a Go-NoGo task devoid of speed constraints, solely focusing on accuracy assessment.
ConclusionIn summary, we could demonstrate that compared to the placebo-feedback group, the neurofeedback group could: (1) increase its SMR power through 10 training sessions of neurofeedback and (2) enhance its inhibition ability at both the behavioral (increased speed and reduced inhibition errors) and neurophysiological (increased NoGoP3d component's amplitude) levels. To model the relationship between the augmentation of SMR power and the increase in NoGoP3d amplitude, our results provided evidence of a positive association, explaining 21 % of the variance.
Building upon our study and previous neurofeedback research, evidence suggest that SMR neurofeedback training holds promise as a tool for rehabilitating inhibition capacities, with the important observation that its effects are supported by substantial distributed changes in cortical representations. Therefore, it is advisable to consider EEG-based neurofeedback when devising rehabilitation strategies for patients suffering from disorders entailing inhibitory impairments; more broadly, it opens possibilities for the management of any neurological and/or psychiatric conditions involving neural losses (Bursky et al., 2022; Deghani et al., 2023; Viviani & Vallesi, 2021).
Limits and future directionsA principal constraint in this investigation is the absence of a positive and significant correlation between the decrease in the number of commission error and the increase in the amplitude of the NoGoP3d component in the NFT group. A second constraint is the lack of baseline raw EEG measurements and the absence of assessments evaluating the effectiveness of the double-blind procedure using metrics such as James' and Bang's indices. Even though we rigorously adhered to the double-blind procedure throughout the experiment, we acknowledge that the variations in amplitude between the different frequency bands that were trained in the placebo group, served as an indicator for making an educated guess regarding the active and placebo conditions. For future perspectives, it would be highly beneficial to conduct an EEG-fMRI study to investigate the neurophysiological processes distinguishing between learners and non-learners, thereby gaining a deeper understanding of how SMR neurofeedback influences cognitive functioning.
FundingThe authors were funded by the Belgian Fund for Scientific Research (F.N.R.S., Belgium) and the Brugmann Foundation (CHU Brugmann, Brussels, Belgium), although these funds did not exert any editorial direction or censorship over any part of this article.
CRediT authorship contribution statementClémence Dousset: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Writing – original draft, Supervision, Project administration, Funding acquisition. Florent Wyckmans: Software, Formal analysis, Visualization, Writing – review & editing. Thibaut Monseigne: Software, Formal analysis. Lauréline Fourdin: Investigation, Writing – review & editing. Romane Boulanger: Investigation, Writing – review & editing. Sonia Sistiaga: Investigation, Writing – review & editing. Anaïs Ingels: Investigation, Writing – review & editing. Hendrik Kajosch: Resources, Project administration, Funding acquisition. Xavier Noël: Conceptualization, Project administration, Funding acquisition. Charles Kornreich: Resources, Project administration, Funding acquisition. Salvatore Campanella: Conceptualization, Methodology, Validation, Writing – review & editing, Resources, Supervision, Project administration, Funding acquisition.