Editado por: Andre R Brunoni, Marie-Anne Vanderhasselt, Leigh Chavert
Más datosNon-invasive brain stimulation (NIBS) techniques have been increasingly used over the dorsolateral prefrontal cortex (DLPFC) to enhance working memory (WM) performance. Notwithstanding, NIBS protocols have shown either small or inconclusive cognitive effects on healthy and neuropsychiatric samples. Therefore, we assessed working memory performance and safety of transcranial direct current stimulation (tDCS), intermittent theta-burst stimulation (iTBS), and both therapies combined vs placebo over the neuronavigated left DLPFC of healthy participants. Twenty-four subjects were included to randomly undergo four sessions of NIBS, once a week: tDCS alone, iTBS alone, combined interventions and placebo. The 2-back task and an adverse effect scale were applied after each NIBS session. Results revealed a significantly faster response for iTBS (b= -21.49, p= 0.04), but not for tDCS and for the interaction tDCS vs. iTBS (b= 13.67, p= 0.26 and b= 40.5, p= 0.20, respectively). No changes were observed for accuracy and no serious adverse effects were found among protocols. Although tolerable, an absence of synergistic effects for the combined protocol was seen. Nonetheless, future trials accessing different outcomes for the combined protocols, as well as studies investigating iTBS over the left DLPFC for cognition and exploring sources of variability for tDCS are encouraged.
Working memory (WM) describes the temporary storage and online manipulation of the information necessary for performing cognitive tasks (Baddeley & Baddeley, 1986). WM is primarily processed in the prefrontal cortex (PFC), particularly its dorsolateral region (DLPFC) (Barbey et al., 2013). Thus, one approach to explore and manipulate WM performance is using non-invasive brain stimulation (NIBS) over this region, such as theta-burst (TBS) or direct current (tDCS) stimulation. The former uses electromagnetic pulses to trigger action potentials and produce long-term changes in cortical activity, being a novel method of transcranial magnetic stimulation (TMS) associated with stronger and faster changes in cortical excitability (Huang et al., 2005). The latter does not trigger action potentials, but rather induces either excitatory or inhibitory changes in cortical activity according to the parameters of stimulation (Nitsche & Paulus, 2000). In fact, although transcranial DCS and TBS can independently modify WM performance (Chung et al., 2018; Moreno et al., 2015), findings have been mixed, and the effects seem to be small, particularly in neuropsychiatric samples. For instance, a recent meta-analysis evaluating the WM performance of health volunteers found only a small positive effect favoring tDCS (Wischnewsk et al., 2021). Similar results were found in another meta-analysis that investigated the TBS effects over the PFC for executive functions (Lowe et al 2018). Moreover, reviews suggest that tDCS over the PFC did not change cognitive performance in neuropsychiatric populations (Martin et al., 2018; Farhat et al., 2020).
Due to these mixed findings innovative NIBS protocols have been pursued such as combining NIBS techniques simultaneously, for achieving larger effects (Hasan et al., 2012). One strategy that has shown promising results in studies investigating the motor and visual cortex excitability was the application of tDCS to preconditing the effects of a secondary NIBS protocol (i.e. tDCS or rTMS). Preliminary studies suggest that preconditioning the target area with tDCS may alter the effects of subsequent rTMS protocols in a manner that generates more robust outcomes (Hurley & Machado, 2017). Similar results were also observed in animal models, in which researchers found that anodal DCS augmented long-term potentiation (LTP) outcomes whereas cathodal DCS seemed to not affect it (Sharma et al., 2021). While these are promising findings, studies combining NIBS protocols in humans have focused primarily on evaluating cortical excitability, a brain outcome that is relatively easier to measure than the ones available for the DLPFC. In fact, only two trials used tDCS to precondition the DLPFC before a course of excitatory rTMS protocol for depression and stress outcomes (De Smet et al., 2021; Loo et al., 2009), but no study investigated the cognitive findings of this combination.
Therefore, here we aimed to evaluate whether working memory performance – as a proxy to DLPFC activity – is modified by application of tDCS, iTBS, or the combination of tDCS + iTBS, in a factorial, double-blinded, sham-controlled trial. Our primary hypothesis was that tDCS and iTBS combined would enhance WM performance. Our secondary hypotheses were that tDCS and iTBS would also improve cognitive performance in comparison to sham. Finally, the tolerability of the NIBS protocols was also investigated. Despite unveiling mechanisms of action of TBS and tDCS on the DLPFC, this would also be important from a clinical perspective; because WM impairment is associated with psychiatric disorders (Bortolato et al., 2014; Gold et al., 2019), and restoring normal WM performance might ameliorate their cognitive symptoms.
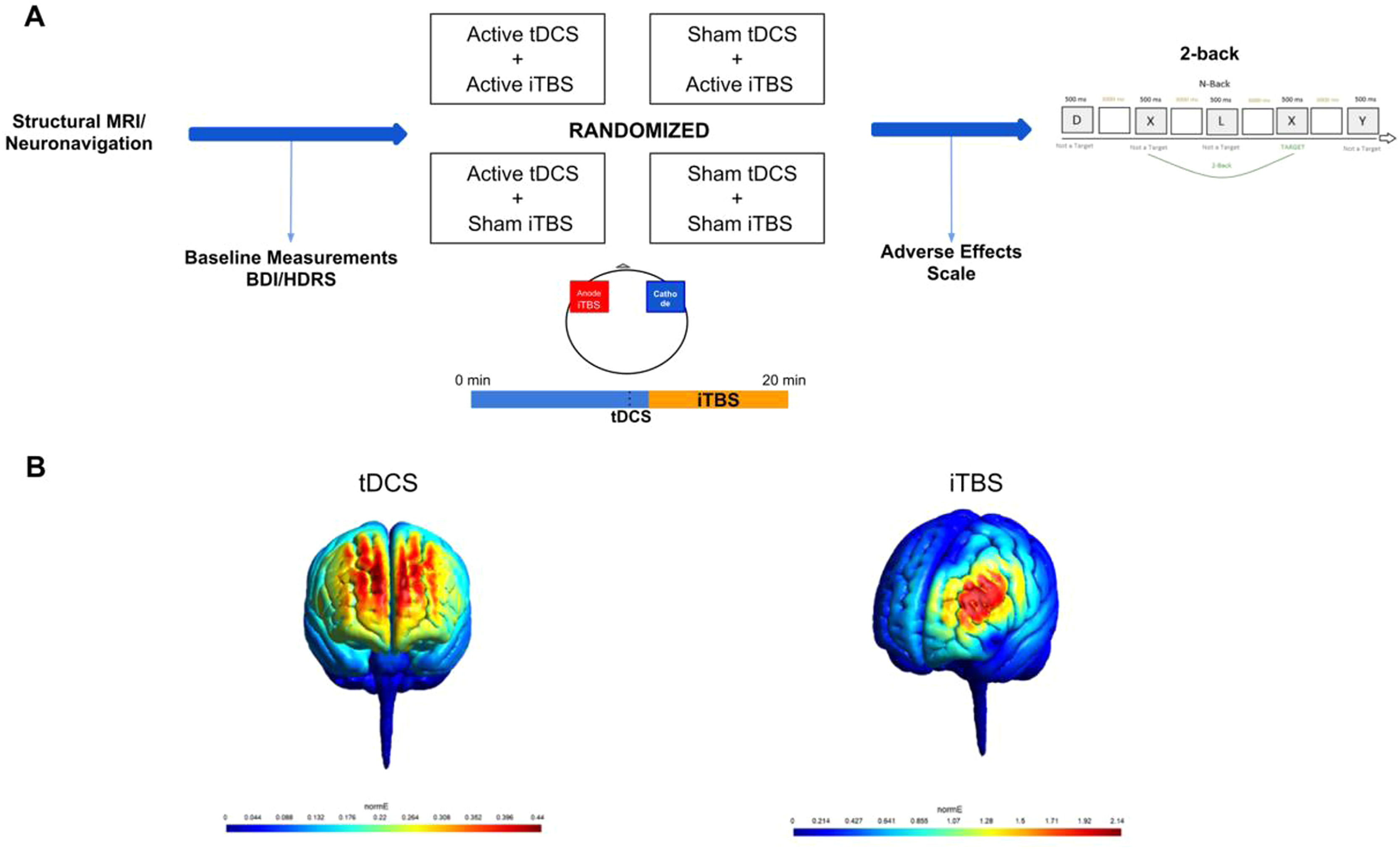
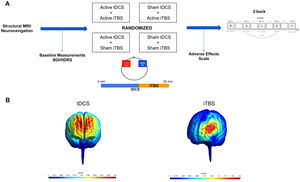
Materials and methodsDesignThe study was conducted at the Institute of Psychiatry, University of São Paulo, Brazil, from March 2019 to March 2021. It was approved by the local and national Ethics Committee (CAAE: 89310918.8.0000.0068) and participants provided written, informed consent. We used a factorial, double-blinded, within-subjects design, in which participants received, once a week and in a randomized order: sham tDCS/sham iTBS (placebo); active tDCS/active iTBS (combined interventions), sham tDCS/active iTBS (iTBS-only); and active tDCS/sham iTBS (tDCS-only) (Razza et al., 2021) (Sup. Material - Appendix A).
ParticipantsWe included right-handed, 18-45 years-old subjects, without previous or current neuropsychiatric disorders or clinical conditions. Participants were recruited through flyers and social media platforms. Volunteers were first screened by e-mail and those who met the inclusion criteria underwent on-site evaluation by a trained psychologist. Exclusion criteria were specific contraindications for NIBS interventions and Magnetic Resonance Imaging (MRI) (e.g., metal implants), current smoker (>10 cigarettes/day), use of substances, pregnancy, and use of psychoactive drugs
ProceduresThe baseline assessment included a brief sociodemographic questionnaire, the Portuguese-based version of the Mini International Neuropsychiatric Interview (MINI) (Amorim, 2000), the Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960), the Beck Depression Inventory (BDI) (Beck et al., 1961).
After these assessments, we collected an anatomical brain scan of the participants in a 3-Tesla MRI equipment (General Electric PET/MRI equipment). In the following days, a neuronavigation procedure (Brainsight, Rogue Resolutions, Inc) was performed, in which we found the left and right DLPFC according to the MNI coordinates x = -38, y = +44, y = +26, per previous studies (Blumberger et al., 2018; Fox et al., 2012).
Each session lasted twenty minutes. TDCS was performed alone for around 11 minutes and TBS was concomitantly applied for the last 8 minutes and 40 seconds. Afterwards, the n-back task was performed, and a questionnaire of adverse effects was applied (Sup. Material - Appendix B) (Aparício et al., 2016). (Figure 1).
(A) Study Design. Structural MRI and the neuronavigation were performed alone in the first session. Afterwards, participants returned to the laboratory for four more sessions, in which different NIBS protocols were applied. TDCS and iTBS were applied concomitantly in all sessions. TDCS electrodes were applied bilaterally over the DLPFC and the iTBS coil was placed on the left DLPFC (over the anode). Baseline measures, adverse effect scale and the 2-back task were applied in all the four experimental sessions. (B) MRI-based computational modeling of tDCS and iTBS. For tDCS, we used a bilateral prefrontal montage with anode placed over the x-38, y+44, z+26 and cathode over the x+38, y+44, z+26, with a current of 2mA and electrodes size of 25cm². For iTBS, we centered the coil at the coordinates x-38, y+44, z+26 with 10mm from the scalp. Computational modeling was performed using SimNIBS (Thielscher et al., 2015).
The tDCS procedure lasted 20 minutes and was applied with a current of 2mA using 25cm² saline-soaked sponges. Anode and cathode were placed over the left and right DLPFCs, as described above. Sham tDCS sessions were identical, but the active current only lasted 30 seconds. The tDCS device was set to deliver either active or sham stimulation according to a randomized code (Neuroconn DC-Stimulator, Ilmenau, Germany).
Regarding iTBS, the coil was placed over the anode and positioned 45° relative to the midline. We performed 54 cycles of 10 triplet bursts with a train of 2s and an interval of 8s between trains (1620 pulses) at 110% of the resting motor threshold, corresponding to a duration of 8 minutes and 40 seconds. The coil used for iTBS has two identical sides that delivers either active or sham stimulation (Cool-B65 Active/Placebo - MagVenture), which is chosen according to a randomized code inputted in the TMS device.
2-Back taskThe cognitive task was programmed in E-prime 2.0 software (Psychology Software, Tools Inc Pittsburgh, Pennsylvania, USA). The visual stimulus consisted of alphabet letters (A to Z) that appeared individually and randomly on a 15-inch screen. Three blocks of 30 letters each, displayed on the screen for 500ms, with an interstimulus interval of 3000 ms, were presented. Each block had 10 target letters – i.e., the same letter that had been presented two letters before. Participants were instructed to identify target and not-target letters by pressing ‘2’ (‘target’) or ‘0’ (‘non-target’), respectively. A brief practice session containing 20 stimuli was performed immediately before each n-back session. The 2-back was chosen because it was previously associated with WM improvement after NIBS in healthy participants (Brunoni & Vanderhasselt, 2014).
Statistical analysisStatistical analyses were performed using RStudio Version 4.1.2 (Team & Others, 2013). Accuracy (binary outcome) and reaction time (RT, in milliseconds (ms)) of the target stimuli were the dependent variables. Missed responses were considered errors. Also, RTs < 200ms and >2500ms were considered not genuine responses and were excluded (Whelan, 2008). Due to the non-normal distribution of RT responses, generalized linear mixed models (GLMM; using the ‘lme4’ package) were calculated to select the statistical model with the best fit. The final model for our analysis was chosen by using the Akaike Information Criterion (AIC) and checking the normality of residuals (See Sup. Material - Appendix C). We employed the inverse-gaussian and binomial distributions for the analyses of RT and accuracy, respectively.
RT or accuracy were the dependent variables, while the factors tDCS (i.e. tDCS yes/ tDCS no) and iTBS (i.e. iTBS yes/ iTBS no), the variable session and their interaction (tDCS*iTBS*Session) were added as fixed effects (Sup. Material - Appendix C). The variable subject was included as a random intercept. Our primary hypothesis would be confirmed if the interaction between tDCS and iTBS were significant, indicating synergistic effects of these techniques. Linear mixed models were conducted to evaluate adverse effects among groups. Pairwise comparisons were post-hoc and performed using the R Package ‘emmeans’. All results were considered significant at a p-value < 0.05. More information regarding sample size calculation can be found elsewhere (Razza et al., 2021).
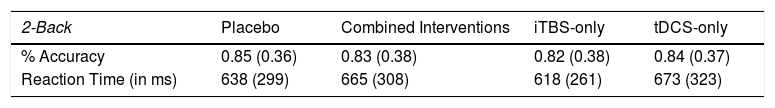
ResultsOut of 211 volunteers, 56 of them underwent onsite or online screening and 24 were included in our study (Sup. Material - Appendix E). Their mean age was 28.7 (standard deviation (SD) = 6.95) years, and most of them were women with university degrees. Twenty-three participants completed all NIBS sessions, with a total of 93 NIBS sessions being performed. The mean accuracy and RT for each group is displayed in Table 1.
Mean and standard deviation of reaction time and accuracy per protocol.
| 2-Back | Placebo | Combined Interventions | iTBS-only | tDCS-only |
|---|---|---|---|---|
| % Accuracy | 0.85 (0.36) | 0.83 (0.38) | 0.82 (0.38) | 0.84 (0.37) |
| Reaction Time (in ms) | 638 (299) | 665 (308) | 618 (261) | 673 (323) |
iTBS: Intermittent theta-burst stimulation; tDCS: transcranial direct current stimulation.
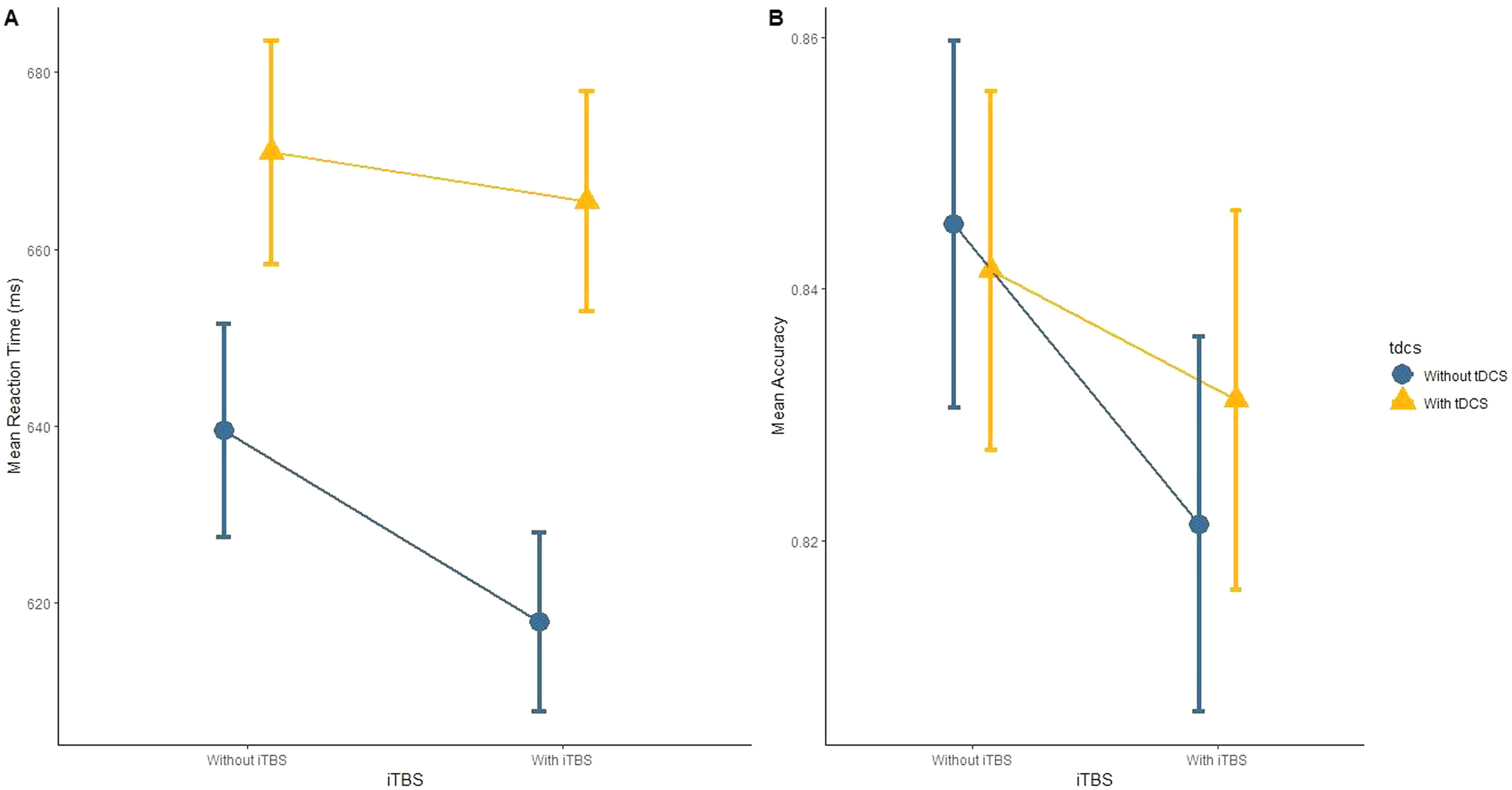
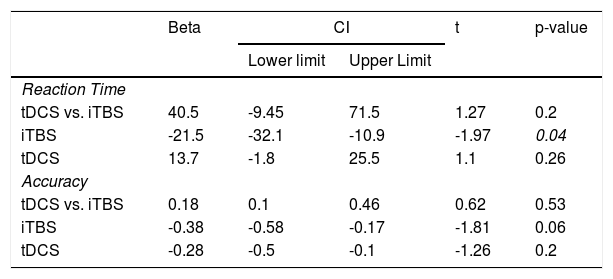
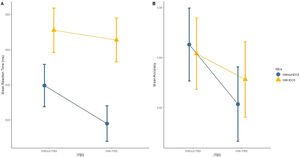
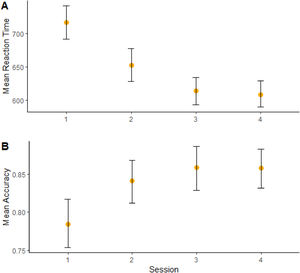
Regarding our primary hypothesis, the factorial analysis revealed no significant effects for the interaction iTBS vs. tDCS for either RT (Figure 2A) or accuracy (Figure 2B) in the 2-back task (Table 2). Analyses “at the margins” revealed that iTBS (iTBS and sham tDCS, and iTBS and active tDCS) presented a faster response than no iTBS (sham iTBS and sham tDCS, and sham iTBS and active tDCS). No effects “at the margins” were found for tDCS.
Main results.
CI: Confidence interval (95%); iTBS: Intermittent theta-burst stimulation; tDCS: transcranial direct current stimulation.
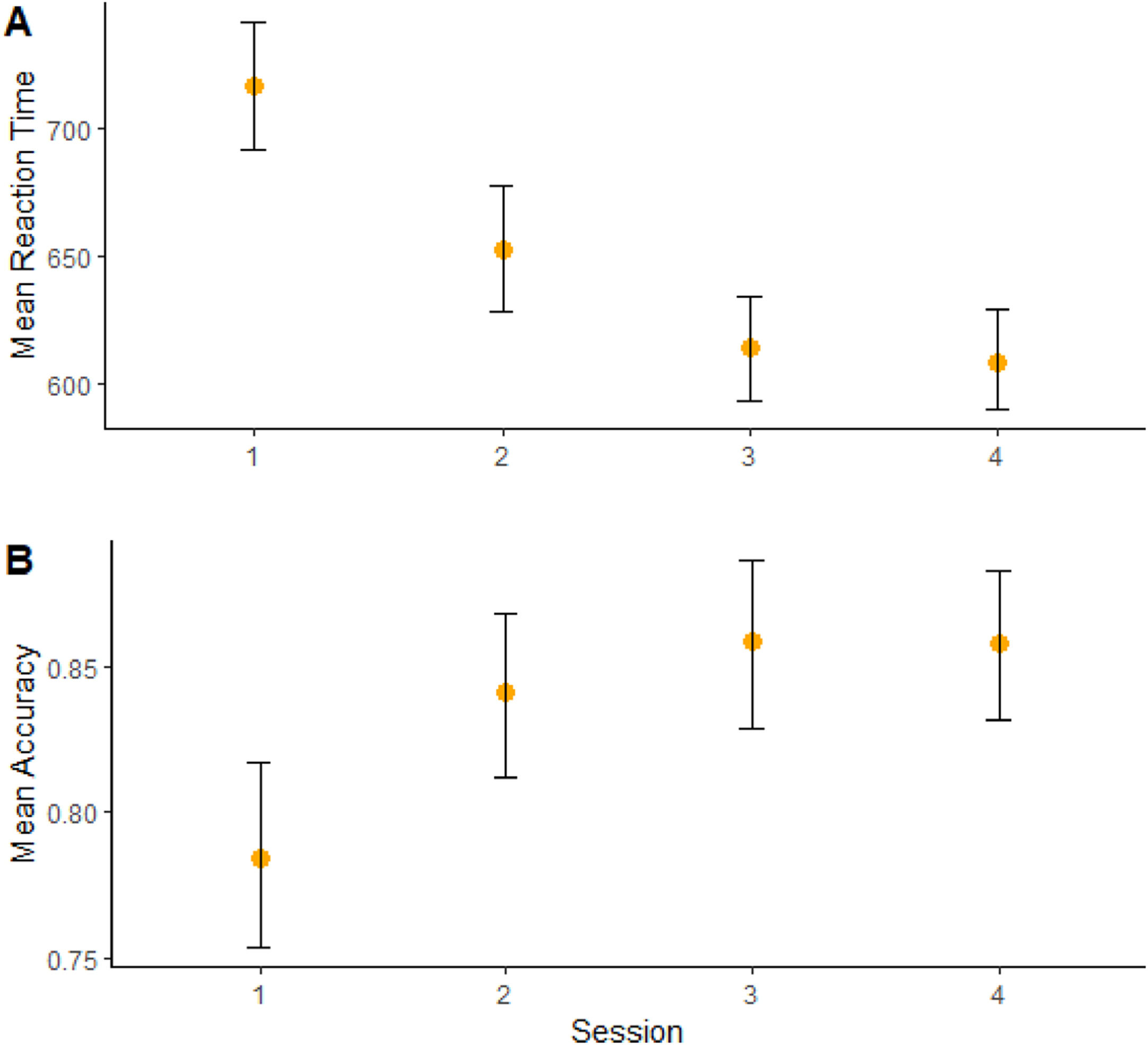
Moreover, a significant effect of “session” on RT (b = -75.3, CI = -88,7; -61.8, t = -5.45, p < 0.001) (Figure 3A) and accuracy (b = 0.56, CI = -0.29; 0.83, t = 2.0, p = 0.04) (Figure 3B) was found, showing cognitive performance increasing over time. (Sup. Material - Appendix F).
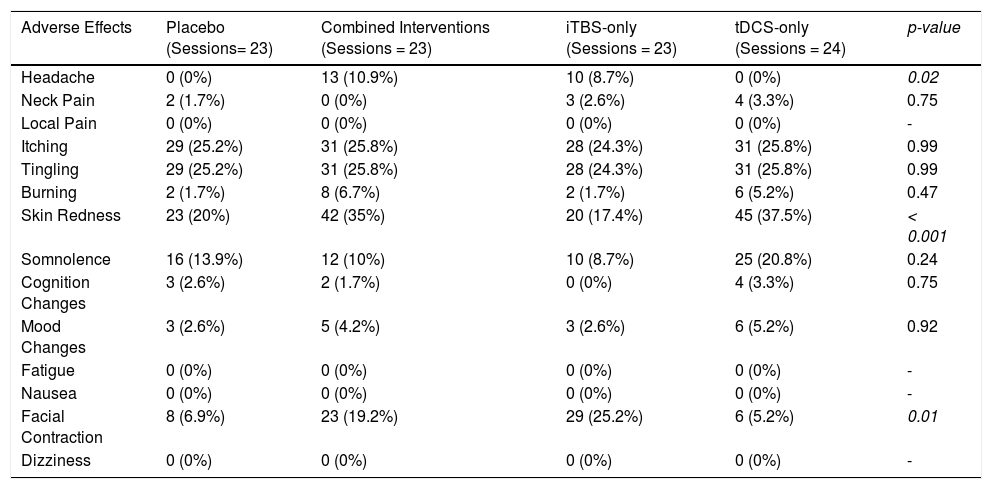
Regarding tolerability, no serious adverse effects, such as seizures, were reported. The combined intervention group presented higher rates of headache compared to placebo and tDCS, but not to iTBS. Participants receiving tDCS presented higher rates of skin redness (Table 3). Finally, facial twitches were more common in the iTBS group (Sup. Material - Appendix G).
Frequency of the adverse effects in each group.
iTBS: Intermittent theta-burst stimulation; tDCS: transcranial direct current stimulation.
The present study was the first to evaluate the cognitive effects of combined and standalone protocols of tDCS and iTBS on the DLPFC in healthy participants. Here, we could not confirm our hypothesis that tDCS and iTBS combined could increase WM performance. Interestingly, we found that iTBS resulted in faster reaction times. Additionally, headache, skin redness, and facial twitches differed among protocols.
Interestingly, previous studies found that the combination of tDCS and iTBS over the motor cortex could enhance motor cortical excitability results (Rubi-Fessen et al., 2015). However, the combination of different NIBS interventions over the DLPFC has been less investigated. In this context, our findings are in line with the results of a recent study that showed no superiority of the combination tDCS-iTBS, compared to iTBS alone, over the DLPFC, on stress response (De Smet et al., 2021). In turn, synergistic effects between tDCS and neurocognitive techniques (Dedoncker et al., 2021) have been previously reported. For instance, the combination of tDCS over the DLPFC with cognitive control training (CCT) and psychotherapy have shown larger effects compared with tDCS alone (Nasiri et al., 2020; Segrave et al., 2014). Differently from NIBS, psychological interventions can engage cortical target areas (Linden, 2006) via behavioral methods. These methods, applied together with tDCS, seem to modulate neural activity in a way that boosts the overall effects more easily (Dedoncker et al., 2021).
Although iTBS and tDCS interventions were applied over the same target, the electric fields generated by each technique present particularities, such as different orientation and strength, which may result in the activation of different cortical areas (Suen et al., 2020; Zaidi et al., 2021). Moreover, we cannot exclude that other intervention protocols – such as cathodal tDCS or continuous TBS – could better improve the effects of each other. For instance, a previous study applying tDCS+iTBS over the motor cortex has shown that preconditioning the brain with tDCS can increase iTBS effects. However, while anodal tDCS significantly reversed the effects of cTBS towards a facilitation increase, it did not affect the iTBS effects (Hasan et al., 2012). Although this could be a rationale behind the non-significant effects of our study, future trials are warranted to investigate the neurobiological effects of this combination over the prefrontal cortex.
Our findings showed that iTBS, but not tDCS, led to significantly faster reaction time. Previous study has already suggested that iTBS can improve working memory performance of healthy volunteers (Hoy et al., 2016). However, to our knowledge, no study compared tDCS and TBS effects within the same design, although a meta-analysis previously suggested an overall greater effect for rTMS than tDCS in improving working memory performance (Brunoni & Vanderhasselt, 2014). Possibly, iTBS enhances endogenous neuronal firing (Suppa et al., 2016) and produces more robust cortical excitability changes in the DLPFC and networks associated with cognition. Moreover, the target method location may have influenced the cognitive effects of iTBS, as recent meta-analyses showed that studies using neuronavigation were associated with higher cognitive performance (Beynel et al., 2019; Pabst et al., 2021).
In contrast, tDCS did not change WM performance in our study. In fact, tDCS findings on cognition have been mixed. For instance, Horvath et al. showed no significant cognitive effects for a single tDCS session vs. placebo in healthy subjects (Horvath et al., 2015). In a recent umbrella review, we found that tDCS could improve cognitive performance, but the quality of the included studies was poor (Farhat et al., 2020). Additionally, tDCS effects over the DLPFC might be non-linear. For instance, a study found that a current intensity of 1 mA – but not 2 mA – improved cognitive performance (Weller et al., 2020). Another issue is the electrode positioning and the corresponding induced electric field over brain areas. For instance, prefrontal, bilateral tDCS montages produce stronger electric fields in the medial prefrontal cortex than the commonly targeted DLPFC (Suen et al., 2020). In this context, it has been proposed that tDCS parameters should be personalized according to the targeted brain region to maximize the induced electric field (Wischnewski et al., 2019). Therefore, further studies investigating optimal tDCS parameters (i.e., position and size of electrodes, current intensity, and others) to increase working memory performance are encouraged.
In our study, learning effects have probably occurred, as we found reaction time decreasing and accuracy increasing over time. This effect has been described in previous studies in the field (Curtin et al, 2019). Nonetheless, our randomized design and statistical analysis might have mitigated this issue.
Although tDCS and iTBS were not associated with any serious adverse event, we observed higher rates of skin redness, headache, and facial twitches after tDCS, the combined treatment, and iTBS, respectively. Such adverse events are consonant with previous studies in the field (Sudbrack-Oliveira et al., 2021; Oberman et al., 2011).
Some limitations should be underscored. First, the sample size was small, which might have increased the rate of false positive and false negative results. Second, accuracy rates were high. As our sample was composed by healthy, educated, and young participants, we probably should have used more challenging versions of the n-back test. Third, as different parameters of iTBS and tDCS produce distinct effects (Brunoni et al., 2019) our findings are limited to those used in our study. Fourth, blinding integrity was not assessed. Finally, in our statistical analysis, RT residuals were not normally distributed, an issue that might have decreased the power of some statistical analyses.
ConclusionThe combination of tDCS and iTBS over the DLPFC of healthy participants was tolerable and did not lead to additive or synergistic effects. Also, effects of iTBS, but not tDCS, indicated an improvement in reaction time, but not accuracy. Our findings encourage future trials evaluating other NIBS parameters and distinct outcomes.
This research project was supported by the São Paulo Research Foundation (FAPESP) (Grant: 2018/10861-7). ARB receives grants from the National Council for Scientific and Technological Development (PQ-1B), and FAPESP (Grants: 2018/10861-7, 2019/06009-6). ARB has a small equity of Flow™, whose devices were not used in the present study. The LIM-27 laboratory receives grants from the Associação Beneficente Alzira Denise Hertzog da Silva. LBR was supported by FAPESP (Grant: 2019/07256-7). MAV received funding from the Research Foundation Flanders (FWO) and from the Ghent University (Grants: G0F4619N and BOFSTA2017002501, respectively). SDS is supported by FWO (Grant:11J7521N).