The presence of bleomycin-induced pneumomediastinum (BIP) in patients with germ cell tumors (GCT) is an uncommon clinical variant within the spectrum of the lung toxicity associated with this drug. The incidence of BIP is currently unknown due to the rarity of this presentation.
Case descriptionWe present the case of a 21-year-old man who presented with good-risk recurrent disease from a germ cell tumor two years after orchiectomy due to disease limited to the testis. BEP was planned for 3 cycles as a treatment schedule. After administration of 30IU of bleomycin, he developed pneumomediastinum associated with respiratory symptoms. After 4 days of surveillance with conservative management, the patient had a good response.
ConclusionsTo our knowledge, we present the first case of BIP after a single standard dose of bleomycin in a patient with testicular cancer. Choosing the patient who would benefit most from bleomycin to perform individualized treatment could reduce the incidence of toxicity associated with this drug in patients with GCT. Special attention should be given to respiratory symptoms during treatment schemes containing bleomycin.
La presencia de neumomediastino inducido por bleomicina (NIB) en pacientes con tumores de células germinales (TCG) es una variante clínica poco común dentro del espectro de toxicidad pulmonar asociada a este fármaco. La incidencia de NIB es actualmente desconocida debido a su rareza.
Caso clínicoPresentamos el caso de un hombre de 21 años de edad que se presentó con recurrencia de un tumor germinal mixto posterior a dos años de vigilancia tras orquiectomía por enfermedad limitada al testículo. Se planteó como esquema de tratamiento el régimen BEP. Tras la primera administración de 30 UI de bleomicina desarrolló neumomediastino aislado. Posterior a 4 días de vigilancia con manejo conservador presentó buena respuesta.
ConclusionesPresentamos el primer caso de NIB tras una sola dosis de bleomicina en un paciente con cáncer testicular. Elegir al paciente que se pudiera beneficiar más con el uso de bleomicina, individualizando su utilización, pudiera reducir la incidencia de toxicidad asociada a este fármaco en los pacientes con TCG. Se debe prestar especial atención a los síntomas respiratorios durante el tratamiento con esquemas que contengan bleomicina.
Bleomycin is an antitumoral drug that belongs to the family of glycopeptide antibiotics isolated from the strain Streptomyces verticillus which is used in patients with a diagnosis of non-Hodkin lymphoma and in individuals who suffer germinal tumors and some squamous carcinomas. Its therapeutic effect is limited due to its potential for lung toxicity, which is known as bleomycin-induced lung injury (BILI), a condition that has a mortality of 1–3%.1
The presence of bleomycin-induced pneumomediastinum (BIP) in patients with germ cell tumors (GCT) is an uncommon clinical variant within the spectrum of lung toxicity associated with this drug. The incidence of BIP is unknown due to the rarity of this presentation, especially in the context of isolated pneumomediastinum. Despite the lack of information regarding this clinical entity, the presence of BIP could be associated with a poorer prognosis and a worse therapeutic outcome in these patients.2
We present the case of a young man with isolated pneumomediastinum associated with a single standard dose of bleomycin.
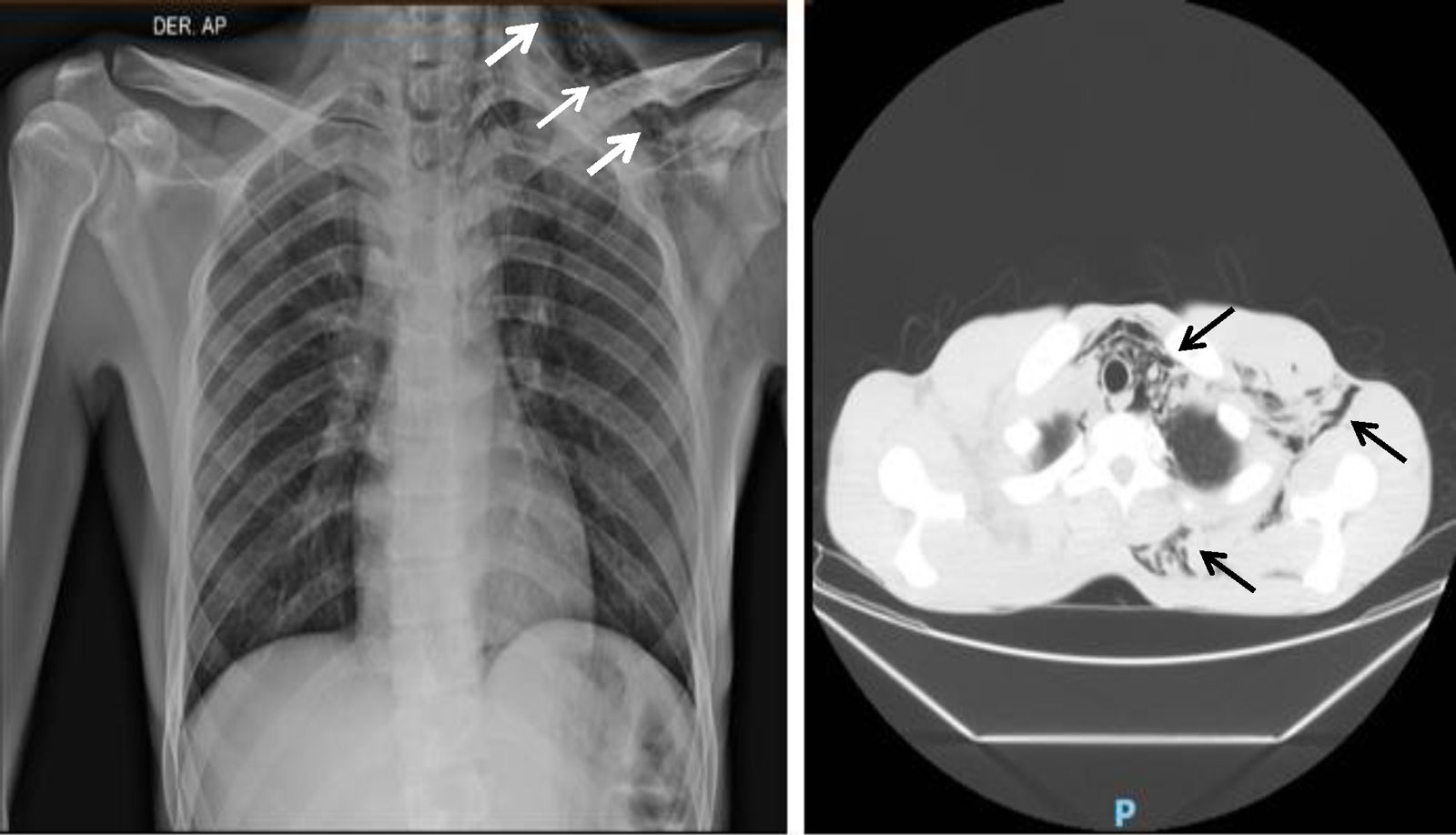
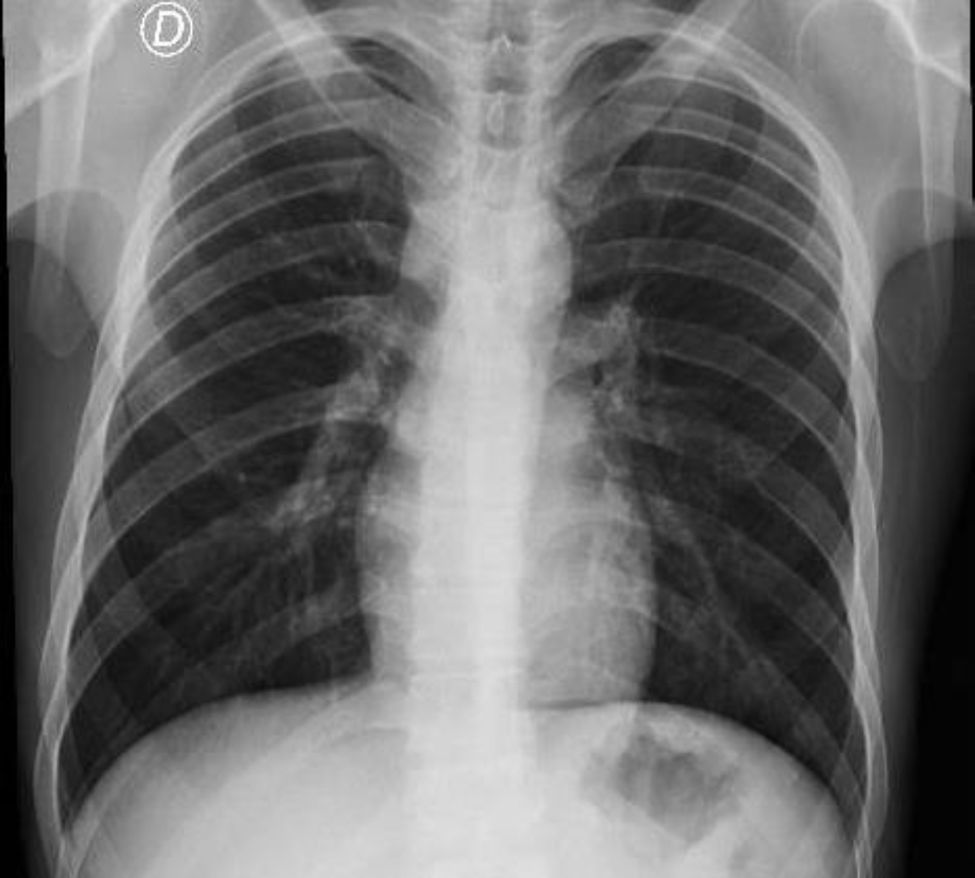
Clinical caseA 21-year-old man came to the emergency department of our hospital complaining of a four-day history of abdominal pain radiating to the right flank. He was diagnosed with a clinical-stage IB right testicular mixed germ cell tumor treated two years before with radical orchiectomy and subsequent surveillance. Imaging studies revealed recurrent metastatic disease to the left inguinal lymph nodes, the mediastinum and the retroperitoneum; the latter causing compression of the right ureter, the inferior vena cava, and thrombosis of the right common iliac vein to the common femoral vein. Unmeasurable disease was documented in relation to bilateral lung involvement. Renal, liver and bone marrow function tests were normal; AFP was 17.28ng/mL (normal range 0–10ng/mL), and B-HCG 1.15mIU/mL (normal values 0–5mIU/mL). The case was initially evaluated by a multidisciplinary team and systemic treatment based on bleomycin, etoposide, and cisplatin (BEP) was offered for three cycles due to good risk features. The first cycle was planned for in-hospital administration. The calculated doses were bleomycin 30IU on days 1, 8, and 15; etoposide 100mg/m2, and cisplatin 20mg/m2 on days 1–5. After the application of the first 30IU of bleomycin, the patient presented sudden pleuritic chest pain located in the left hemithorax and associated with dyspnea at rest. On physical examination, subcutaneous emphysema was detected in the ipsilateral neck and supraclavicular region. Imaging studies revealed pneumomediastinum and subcutaneous emphysema without pneumothorax (Fig. 1). Conservative management was provided with supplemental oxygen; clinical and radiographic resolution occurred after 4 days of observation (Fig. 2). After monitoring with a favorable evolution, hospital discharge was authorized. In the patient's first outpatient assessment, it was decided to suspend bleomycin and change the treatment scheme to etoposide and cisplatin (EP) at standard doses.
The clinical presentation of BIP is variable and includes a wide spectrum of thoracic symptoms such as dyspnea, cough, chest pain, rales, opacities on imaging studies, such as chest X-ray or CT scan, and decreased lung capacity detected by studies, such as the diffusing capacity of the lungs for carbon monoxide (DLCO). BIP can occur as early as the first month of treatment or as a late pulmonary toxicity 10 years after the end of bleomycin treatment as described by Tashiro et al.3 Lung toxicity from bleomycin has been related to the activity of the enzyme bleomycin-hydroxylase which deactivates this drug. Genetic variability in its activity has been found in some studies,4 leading to greater susceptibility in patients who have a lower function of this enzyme for the development of an inflammatory mechanism mediated by cytokines and free radicals of endothelial damage of the lung vasculature with the subsequent clinical picture manifested as BILI.5 Renal failure, a body mass index (BMI) less than 22kg/m2, the use of granulocyte colony-stimulating factor, tobacco use, the presence of prior interstitial lung pathology, a cumulative dose greater than 300IU of bleomycin, previous irradiation or involvement of the lung parenchyma due to metastatic lesions have been described in the literature as risk factors for the development of BILI.6–9
Most of the literature and the current medical review of this clinical presentation concludes as a major precipitant a greater amount of accumulated dose of bleomycin, mostly with the late development of concomitant interstitial pneumopathy10 together with multiple pulmonary metastatic lesions with high chemosensitivity as occurs in testicular germ cell tumors9; however, the relevance of this clinical case is the atypical presentation where pneumomediastinum developed after the first dose of 30IU.
To our knowledge, we present the first case of BIP after one standard dose of bleomycin. We encourage future trials with statistical power to predict which patients will benefit the most from bleomycin and therefore reduce the incidence of toxicity in those who will not have a significant benefit from this drug. The physician must consider the probability of BILI within the initial approach of a patient with any pulmonary symptom during treatment with bleomycin; this will facilitate timely diagnosis in order to avoid life-threatening complications.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare that there is no conflict of interest regarding the publication of this paper.
Peer-review under responsibility of the scientific committee of the International Conference on Women and Societal Perspective on Quality of Life (WOSQUAL-2019). Full-text and the content of it is under responsibility of authors of the article.