To evaluate the acute wound healing potential of RDFE cream on type III collagen and EGF levels.
MethodMale Albino Wistar rats have randomly divided into three groups: negative control received base cream, positive control administered with 10% zalf povidone and RDFE group was treated with RDFE cream. The healing effects were assessed on EGF, type III collagen, wound diameter, tissue granulation, and epithelization, for 3, 7, 11, and 14 days post-treatment.
ResultsOn the 3rd-day post-treatment, the greatest reduction in wound diameters were shown significantly in the positive control among groups (p<0.05). Although the data were statistically insignificant, RDFE groups showed the highest level of type III collagen among groups after 7-day treatment (p>0.05).
ConclusionThe effect of RDFE cream in the wound healing process on type III collagen appeared on day 7. However, an increase of EGF levels was not observed following RDFE treatment.
Globally, more than 305 million acute wounds are recorded per year. The number of the recorded acute wound is more than nine times of the total number of people around the world living with cancer.1 Thus, treatment approaches are urgently needed to overcome the high number of acute wounds. Various attempts are used to accelerate the wound healing process. However alternative/complementary using natural compounds are highly demanded.2 Also, the topical application of medication is preferred due to the lack of adverse effect on other organs.3
Plants are the greatest sources of new compounds which have properties including antimicrobial, antiviral, antioxidants, and the others. According to WHO, the properties of plants provide a way to combat diseases.3 Red dragon fruit is one of the fruits which was grown in Indonesia. The natural ingredients contained in the Red Dragon Fruit have potential in the wound healing process. In Indonesia, the effect of red dragon fruit extract (RDFE) on wound healing has been explored in the last three years.4
The healing process of wounds is an interaction of cellular structure reconstruction and multiple coordinated cascades of cellular and molecular events, which leads to tissue repair.3 The interaction of cytokines, which have mitogenic, pro-inflammatory and anti-inflammatory activities, and proteases, are playing a role in each stage of the healing process.5 EGF is one of the growth factors which plays a main role in the epithelialization process.6 Collagen also plays an important role in the proliferation process. It is secreted to the extracellular space in the form of pro-collagen and then cleaved from the tropocollagen.7 This tropocollagen forms a strong cross bond, making collagen fibers resistant to damage. And the more these cross-links form in intramolecular and intermolecular collagen, the stronger the wound healing becomes.8 The importance of the role of EGF and collagen make those become the sensitive biomarker of the wound healing process. Although many studies have been conducted on RDFE, its potential effects on EGF and collagen have not been explored yet. Therefore, this study aimed to evaluate the wound healing potential of RDFE cream on EGF and type III collagen levels.
MethodSeventy two healthy albino male Wistar rats aged 6–8 weeks weighing 250–350g were obtained from Gajah Mada University, Yogyakarta, Indonesia, then were randomly divided into 3 groups: (1) the positive control group (n=24) administered with 2-mg 10% povidone-iodine cream, (2) the negative control group received 2-mg base cream, and (3) RDFE group was treated with 2-mg RDFE cream. Afterward, each group was divided into four subgroups of 6 animals supervised for 3, 7, 11, and 14 days post-treatments. The experimental procedures were performed with the approval of The Research Ethics Committee of the Faculty of Medicine, Hasanuddin University (No 400/H4.8.4.5.31/PP36-COMETIC/2018).
The rats were anesthetized by inhalation using isoflurane (0.01–0.05μg/kg) before wound infliction.9 The excision area (left and right back) were firstly shaved using a hair remover (Jollen) and cleaned with chlorhexidine 0.5%+alcohol 70%.9 The wound was made with a biopsy punch of 8mm in diameter.10 The protocol of this study refers to the Council for International Organizations of Medical Sciences (CIOMS).11
The red dragon fruits were purchased from the plantation in Samarinda, East Kalimantan, Indonesia. The fruits were extracted using methanol, then the percentage of free radical inhibition (DPPH) test was conducted. The anti-free radical activity of RDFE and vitamin C obtained IC50 values, respectively 314.69ppm, and 3.28ppm. Every 1 gram of RDFE contains 1062 GAE of polyphenols and 8.3mg flavonoid. 7.5% concentration of RDFE were used to make the topical RDFE cream.
On the 3rd, 7th, 11th, and 14th day, the measurement of wound diameter were performed using a slide/wound ruler. On the respective days, skin tissue fragments containing the wound with a margin of 1cm were collected. Those withdrawn from the left-back were fixed in 10% normal formalin buffer solution for further histopathological assessment, while those from the right-back were immediately stored in 0.9% NaCl buffer for further ELISA analysis.
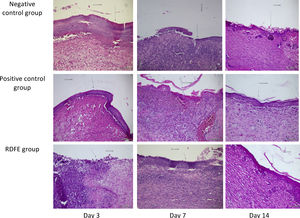
The skin samples fixed in 10% normal formalin buffer solution, were dehydrated with various concentrations of alcohol (70%, 80%, 90%, and absolute alcohols I and II), cleared by xylol and embedded in paraffin. The tissues were put into a liquid paraffin printer and left until the paraffin hardens. The tissues then were cut into 5-μm-thick sections using a microtome. The tissue sections were rehydrated and stained with hematoxylin–eosin (HE). The tissue granulation thickness (proliferation) and connective tissue area were measured using a JVC, Japan micrometer with four times magnification. The epithelial scores and tissue granulation thickness were evaluated. The semi-quantitative method of histological sections was used to assess the epithelization of the wound.12 Meanwhile, the thickness of tissue granulation was assessed using the histological score, in which the score of 1 (thin), score of 2 (moderate), and a score of 3 (thick) are used.13
The skin fragments were homogenized with sonicator, and supernatants were collected. Quantitative measurements of EGF levels was performed using a commercial enzyme-linked immunosorbent assay (ELISA) kit (EGF mouse kit Immunoassay Quantikine kit; MEG00) according to the manufacturer's instructions. Meanwhile, type III collagen levels were measured using ELISA kit (Mouse Collagen III; E0338, Shanghai, China) according to the manufacturer's instructions.
One-way analysis of variance (ANOVA) and Kruskal–Wallis tests was carried out to identify the different effects between treated groups and control groups using SPSS Ver.21 (SPSS Inc., Chicago, IL, USA).
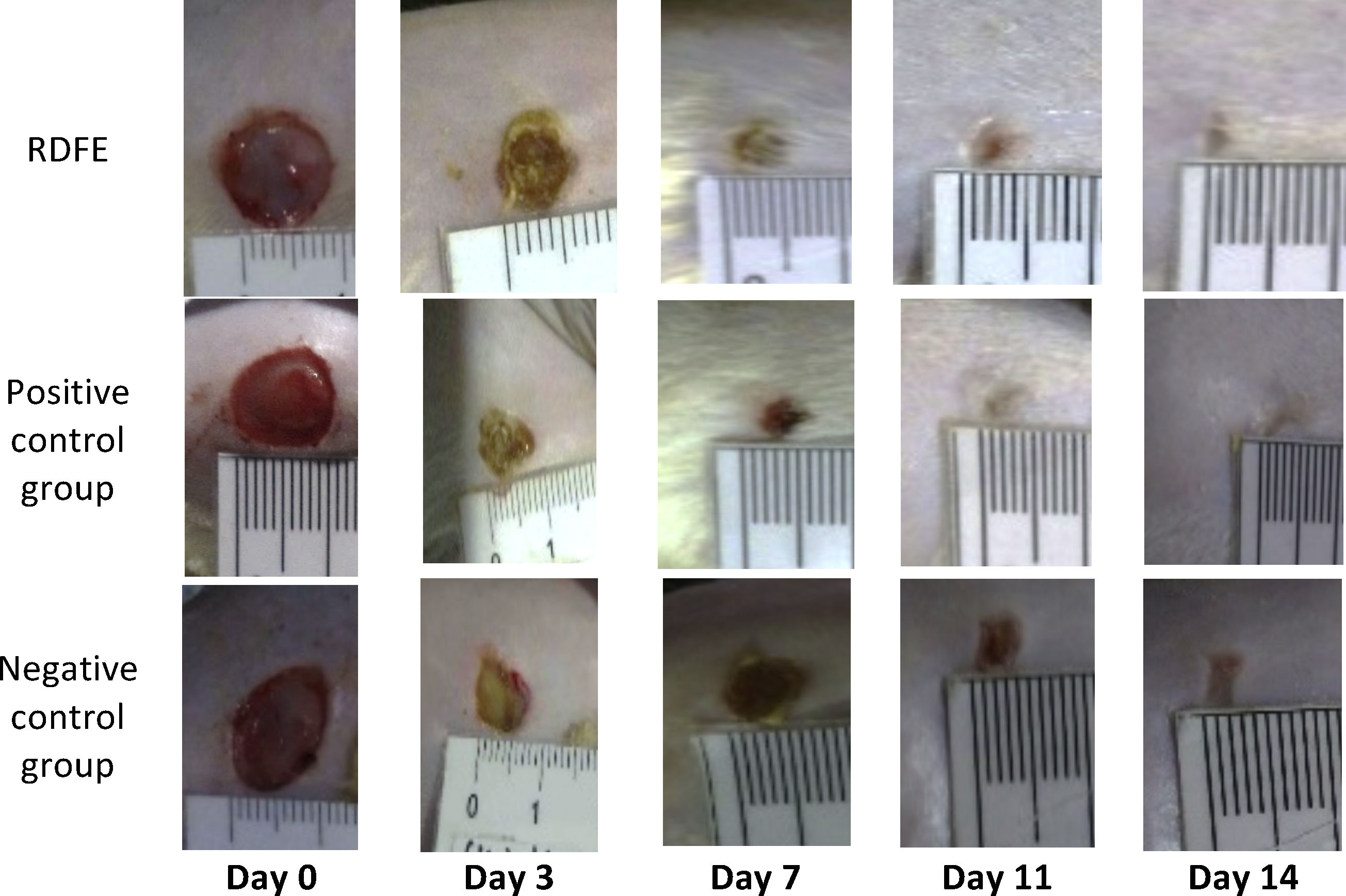
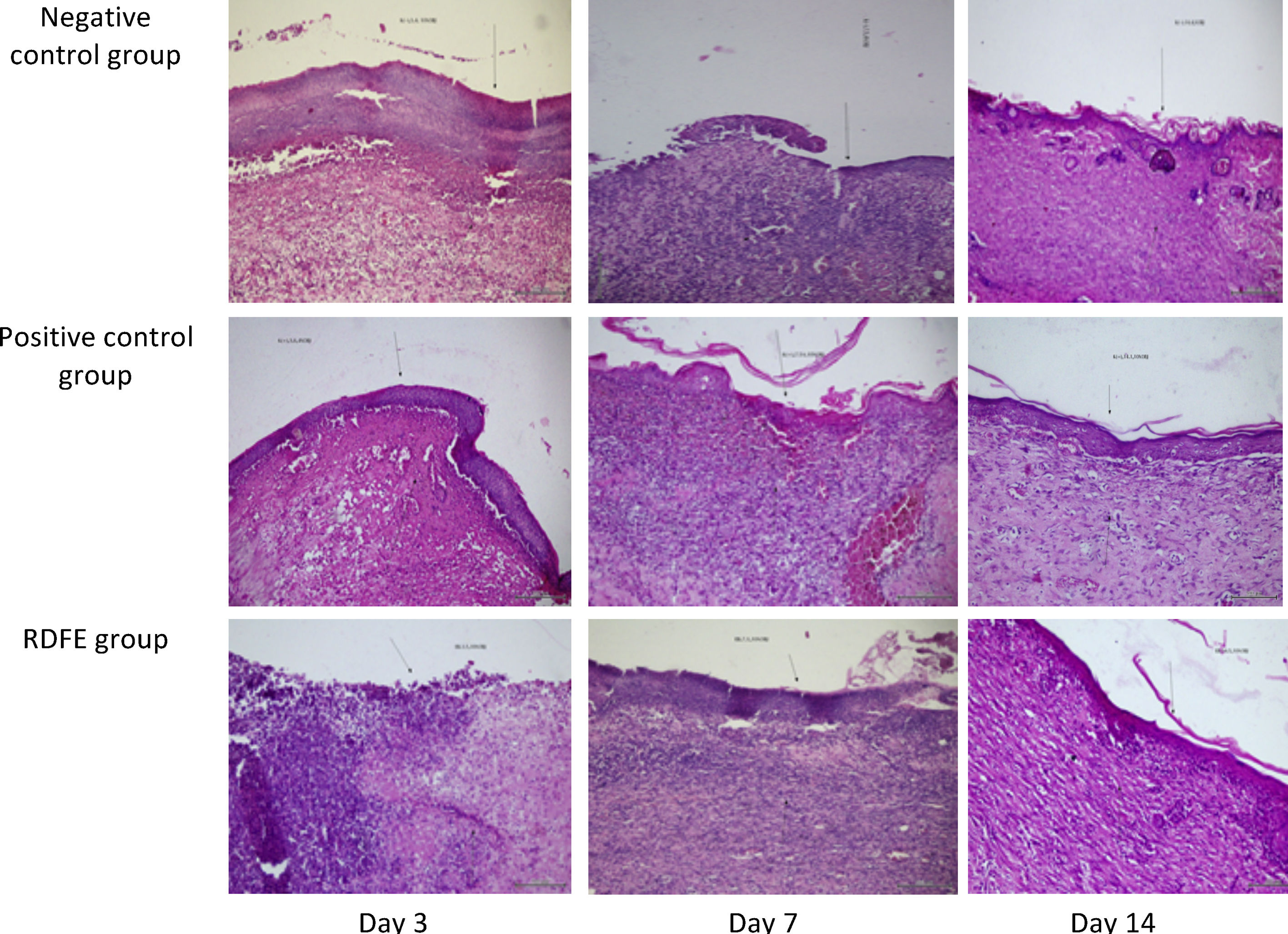
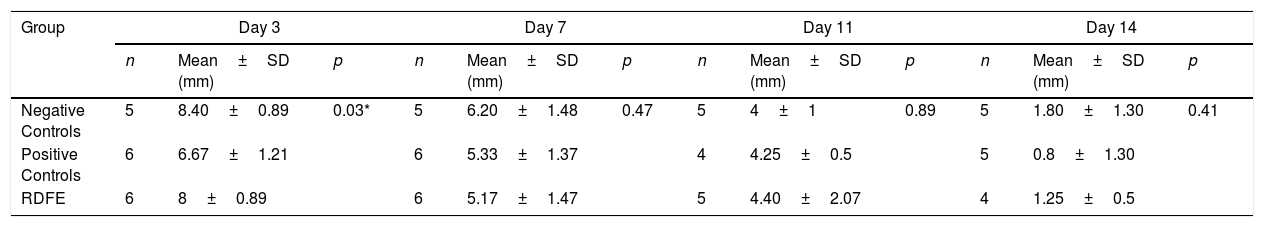
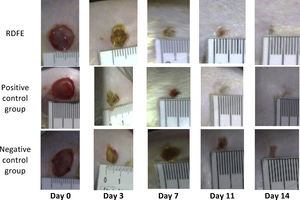
ResultsOn day 3, the smallest wound diameters were shown significantly in the positive control among groups (p<0.05). After seven days of treatment, the RDFE group showed the smallest diameter among groups, although the data were statistically insignificant (p>0.05). Meanwhile, on day 11 after treatment, the smallest diameter of the wound was shown in the negative control among groups (Table 1), but the differences were not statistically significant (p>0.05). On day 14, the positive control showed the smallest diameter of wound among groups, but no statistical significance was found (p>0.05). The macroscopic descriptions of wound diameter reduction were presented in Fig. 1. Positive control groups indicated the thinner granulation tissue on day 14 compared to other groups. In the re-epithelialization tissue, the epithelial closure appeared better on positive control group among groups on day 14 (Fig. 2).
Wound diameter evaluation in the different treatment group.
| Group | Day 3 | Day 7 | Day 11 | Day 14 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean±SD (mm) | p | n | Mean±SD (mm) | p | n | Mean±SD (mm) | p | n | Mean±SD (mm) | p | |
| Negative Controls | 5 | 8.40±0.89 | 0.03* | 5 | 6.20±1.48 | 0.47 | 5 | 4±1 | 0.89 | 5 | 1.80±1.30 | 0.41 |
| Positive Controls | 6 | 6.67±1.21 | 6 | 5.33±1.37 | 4 | 4.25±0.5 | 5 | 0.8±1.30 | ||||
| RDFE | 6 | 8±0.89 | 6 | 5.17±1.47 | 5 | 4.40±2.07 | 4 | 1.25±0.5 | ||||
RDFE: Red Dragon Fruit Extracts. Asterisk (*) indicates significantly different p<0.05 as compared to negative controls and RDFE groups.
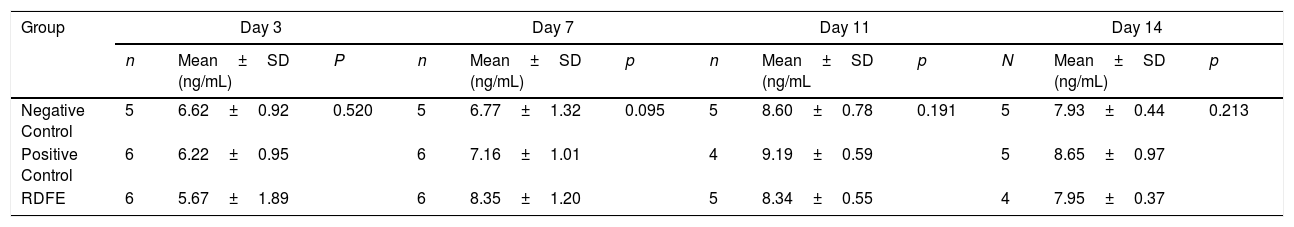
After 3-day treatment, type III collagen levels were found lower in the RDFE group compared to other groups, but the differences were statistically insignificant (p>0.05). RDFE groups showed the highest level of type III collagen compared to another group after 7-day treatment (p>0.05). However, after 11 and 14-day treatment, the highest levels of type III collagen were shown in positive control groups, as shown in Table 2.
The Effect of RDFE cream on type III collagen levels.
| Group | Day 3 | Day 7 | Day 11 | Day 14 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean±SD (ng/mL) | P | n | Mean±SD (ng/mL) | p | n | Mean±SD (ng/mL | p | N | Mean±SD (ng/mL) | p | |
| Negative Control | 5 | 6.62±0.92 | 0.520 | 5 | 6.77±1.32 | 0.095 | 5 | 8.60±0.78 | 0.191 | 5 | 7.93±0.44 | 0.213 |
| Positive Control | 6 | 6.22±0.95 | 6 | 7.16±1.01 | 4 | 9.19±0.59 | 5 | 8.65±0.97 | ||||
| RDFE | 6 | 5.67±1.89 | 6 | 8.35±1.20 | 5 | 8.34±0.55 | 4 | 7.95±0.37 | ||||
RDFE: Red Dragon Fruit Extracts.
After cream treatment for three days, the clinical EGF levels were highest in positive controls, compared to the negative controls and RDFE groups. However, no statistical significance was found (p>0.05). On day 7 and 11 after treatment, RDFE groups showed the highest level of EGF compared to positive and negative controls, although the differences were not statistically significant. Finally, on day 14, after treatment, the EGF levels were shown higher in positive controls, compared to RDFE and negative controls, as shown in Table 3.
The Effect of RDFE cream on EGF levels.
| Group | Day 3 | Day 7 | Day 11 | Day 14 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median(Min–Max) (ng/L) | p | n | Median(Min–Max) (ng/L) | p | n | Median(Min–Max) (ng/L) | p | n | Median(Min–Max) (ng/L) | p | |
| Negative control | 5 | 110.74(104.27–162.49) | 0.25 | 5 | 109.63(108.95–160.11) | 0.08 | 5 | 159.08(152.19–164.87) | 0.67 | 5 | 153.98(123.17–182.75) | 0.96 |
| Positive control | 6 | 138.32(100.61–156.53) | 6 | 152.79(148.02–168.53) | 4 | 162.70(157.30–174.32) | 5 | 157.55(145.98–164.53) | ||||
| RDFE | 6 | 102.61(96.44–167.68) | 6 | 169.38(129.98–176.96) | 5 | 163.34(131.93–164.34) | 4 | 156.61(148.28–162.15) | ||||
RDFE: Red Dragon Fruit Extracts.
Our study is the first study that examines the potential effect of RDFE on acute wound healing in healthy subjects. The main findings of the present study were that the significant wound diameter reduction was observed on day three post-treatment, while on the 7th day, the peak effects of RDFE were shown on wound diameter compared to other groups. However, on the 14th day, greater reduction in wound diameter appeared in RDFE group, compared to other groups. The reduction of wound diameter might result from the presence of flavonoids and phenol as anti-inflammatory components in RDFE. RDF also contains quercetin and tannin, which play a role in the wound healing process.14 A study conducted by Kant et al. (2017) revealed that the topical application of 0.1% quercetin accelerates the closure of wound compared to the negative control.15 We assume the quercetin in RDFE cream contributes to wound closure through promoting the angiogenesis and proliferation of epithelial cell and fibroblast.15
The present study showed that RDFE cream containing flavonoid contributed to increasing the production of type III collagen. The highest peak of type III collagen levels was observed on day seven post RDFE treatment, although the difference between groups was statistically insignificant. During the initial wound healing, type III collagen is the main collagen produced by fibroblasts in granulation tissue during the proliferation phase. It firstly appears after 48–72h and is maximally secreted between 5 and seven days.16 The number of collagen was increased at the beginning of the repair phase, reaching a maximum between 2 and 3 weeks after injury.17 Many studies using natural ingredients containing flavonoids have been shown to increase collagen synthesis in wound healing. Therefore we assume the content of flavonoids in RDFE cream might contribute to the process of wound healing through an increase of collagen synthesis.
Our study found that there were no statistically differences in EGF level between groups. Our finding was not by the findings of the previous studies evaluating the growth factors. Djamaluddin (2016) indicated the elevation of FGF levels in RDFE group in wound proliferation phase.18 There are several studies on cytokines that play a role in the wound healing process, for instance, MMP-9 levels after 14 days of treatment were shown to decrease in the topical RDFE group.19 The other findings revealed that IL-6 levels in the RDFE group were lower compared to the group treated with base cream.20 RDF was also reported to contain vitamin E.21 Butt et al. (2017) who examined in vitro human dermal fibroblasts indicated vitamin E induced a significant increase in paracrine release of EGF.22 The different findings in several studies might result from differences in the type of RDFE, type of Wistar, body weight, age, and time of wound measurement. We also assumed that the vitamin E content in RDFE cream might be insufficient to induce a significant increase in EGF levels.
ConclusionsThe wound healing potential effect of RDFE cream on type III collagen was appeared on day 7. However, an increase of EGF levels was not observed following RDFE treatment. Therefore, further researches on a higher concentration of RDFE are needed to investigate the optimal concentrations of RDFE, which can induce the optimal wound healing.
Conflict of interestThe authors declare no conflict of interest.
Authors would like to thank the Animal Laboratory, Anatomical Pathology Laboratory of Hasanuddin University and Indonesian Muslim University.
Peer-review under responsibility of the scientific committee of the International Conference on Women and Societal Perspective on Quality of Life (WOSQUAL-2019). Full-text and the content of it is under responsibility of authors of the article.