This study aims to evaluate the effect of electrolyzed water effect on bacterial colonization on diabetic foot ulcer (DFU) patients.
MethodThis is an in vitro study. Eight bacteria are incubated into 5 types of electrolyzed water (ESAW), electrolyzed weak acid water (EWAW), electrolyzed neutral water (ENAW), electrolyzed weak alkaline water (EWAlW), and electrolyzed strong alkaline water (ESAlW). Evaluations were performed 2, 24, 48 and 72h after incubation. Data were analyzed using repeated ANOVA test used to compare the difference of electrolyzed water effect on bacterial colonization of DFU patients.
ResultsESAW (pH 2.5) significantly (p=0.001) had a better bactericidal effect than EWAW, ENAW, EWAlW and ESAlW.
ConclusionCurrent study confirmed the positive effect ESAW on bacterial colonization in DFU patients.
Diabetic foot ulcers (DFUs) associated with various devastating complications, such as; long term care, high cost,1,2 infection,3 amputation4,5 and mortality.6 Therefore prompt and aggressive treatment are needed to accelerate wound healing progress, which will be prevent worsening of ulcers, maintain extremity intact and quality of life.4
One of essential modality in management of DFUs is wound cleansing. Wound Cleansing is one of wound care procedure.7,8 At least normal saline and tap water are reported as main wound cleansing agent wound cleansing,9 meanwhile the advantages of acid cleansing has less evaluated.
Previous study revealed that Hypochlorus acid improved wound healing since it has antimicrobial effect including in DFUs.10 The benefits of pH neutral in a study of Neutralized electrolyzed water (NEW) effects that were preceded by an in vitro study showed that NEW pH 7.7 significantly reduced the number of Aspergillus flavus spores and Penicillium expansum spores.11 Although electrolyzed alkaline water has many health benefits including improving abnormal intestinal fermentation, chronic diarrhea, gastric hyperacidity, dyspepsia, improving constipation, suppressing body fat accumulation, expelling early melamine, reducing skin damage due to ultraviolet light, modulation of immune responses and repair diabetes.12 Despite many studies has reported the benefit of pH water, the effectiveness as wound cleansing remain unknown. Thus, the aim of current study to evaluate the effect of electrolyzed water effect on bacterial colonization on diabetic foot ulcer (DFU) patients.
MethodsSettingThis was an experiment in vitro study. The swabbing were taken from DFUs patients both inpatient-outpatient clinic (Hospital) and three home care setting (private wound care practices) by an accidental sampling technique. HbA1C>6% and Texas university classification system (grade B), as inclusion criteria.
Swabbing were taken using Levine technique. Culture of wound specimens through inoculation on the medium, incubated for 24h to identify bacteria and its gram properties.
PreparationThe electrolysis process of water uses the electrolysis machine (Leveluk SD 501, Japan), to prepare five different types of electrolyzed water (EW). Status of EW were measured by pH meter (Hanna pHep Tester HI98107, Romania), and validated at the Makassar Environmental Health and Disease Control Laboratory.
In vitroThe identified bacteria were made suspended in 0.9% NaCl solution using Mcfarlan 0.5 standard, then insert 5 types of electrolyzed water (EW) into the 9ml screw tube, sterilize. Suspension of 8 types of bacteria 1ml were added into a tube which each contains 5 types of EW, then mixed until homogeneous. Incubation running for 2h then put into sterile petri dish and adding medium plate count agar (PCA) to the petri dish, mix until homogeneous. After PCA solidified, incubate it into an incubator at 37°C for 2h. After 2h incubation, the number of bacterial colonies that grow on the medium were calculated. The procedure were repeated until the second day (48h) and third (72h).
AnalysisDemography data presented in frequency table (n, %), while evaluation of electrolyzied watered against bacterial colonization were analyzed using repeated one way anova (SPSS, ver 22, Chicago Inc). Ethical clearance was approved from Ethical Committee, Faculty of Medicine, Hasanuddin University. No. 437/H4.8.4.5.31/PP36-KOMETIK/2017.
ResultsCharacteristics of participantsHalf of participants on early elderly on age group (46–55 years) (n: 10, 50.0%), the mean and standard deviation of the participants’ age (53.1, ±6.7), dominantly male gender (n: 14, 70.0%), Islam (n: 19, 95.0%), Bugis ethnic (n: 12, 60.0%), the last education for Senior High School (n: 8, 40.0%), government officer and self-employed (n: 8, 40.0%) (Table 1).
Frequency distribution of participants based on demographic data.
| Variabel | Total | |
|---|---|---|
| n: 20 | % | |
| Age (mean, +SD) | 53.1 | 6.7 |
| Late adults (36–45 years old) | 3 | 15.0 |
| Early elderly (46–55 years old) | 10 | 50.0 |
| Late elderly (56–65 years) | 7 | 35.0 |
| Gender | ||
| Male | 14 | 70.0 |
| Female | 6 | 30.0 |
| Religion | ||
| Islam | 19 | 95.0 |
| Christian Protestant | 1 | 5.0 |
| Ethnic | ||
| Bugis | 12 | 60.0 |
| Makassar | 6 | 30.0 |
| Toraja | 1 | 5.0 |
| Java | 1 | 5.0 |
| Last education | ||
| Primary School | 3 | 15.0 |
| Junior High School | 1 | 5.0 |
| Senior High School | 8 | 40.0 |
| Diploma | 2 | 10.0 |
| Bachelor/Masters/Doctorate | 6 | 30.0 |
| Occupation | ||
| Government officer/Army/Police/Lecture | 8 | 40.0 |
| Honorary | 1 | 5.0 |
| Entrepreneur | 7 | 35.0 |
| Farmer | 1 | 5.0 |
| Housewife | 3 | 15.0 |
DM status based on diabetes duration <5 years (n: 8, 40.0%), mean and standard deviation of HbA1C (10.3, ±2.6%), Glycemic Control by oral (n: 8, 40.0%), history of never smoking (n: 8, 40.0%), the mean and standard deviation of the participants’ height (165.9cm, ±7.4), weight (62.9kg, ±10.1), Body Mass Index (BMI) (22.7kg/m2, ±2.4), the normal BMI category (18.50–24.99) (n: 16, 80.0%), systolic blood pressure (130.1mmHg, ±19.5), and diastole blood pressure (84.5mmHg, ±12.8) (Table 2).
Frequency distribution based on the participants’ diabetes mellitus status.
| Variabel | Total | |
|---|---|---|
| n: 20 | % | |
| Duration of diabetes | ||
| <5 years | 8 | 40.0 |
| 5–10 years | 5 | 25.0 |
| >10 years | 7 | 35.0 |
| Glycemic Control | ||
| There is no | 3 | 15.0 |
| Oral | 8 | 40.0 |
| Insulin | 5 | 25.0 |
| Oral and insulin | 4 | 20.0 |
| Smoking history | ||
| Never | 8 | 40.0 |
| Ever | 8 | 40.0 |
| Active | 4 | 20.0 |
| HbA1C (mean, ±SD) | 10.3 | 2.6 |
| Blood pressure | ||
| Sistole (mmHg) (mean, ±SD) | 130.1 | 19.5 |
| Diastole (mmHg) (mean, ±SD) | 84.5 | 12.8 |
| Height (cm) (mean, +SD) | 165.9 | 7.4 |
| Weight (Kg) (mean, +SD) | 62.9 | 10.1 |
| Body Mass Index (BMI) (kg/m2) (mean, +SD) | 22.7 | 2.4 |
| BMI category | ||
| Underweight (<18.49) | 1 | 5.0 |
| Normal (18.50–24.99) | 16 | 80.0 |
| Overweight (25.00–29.99) | 3 | 15.0 |
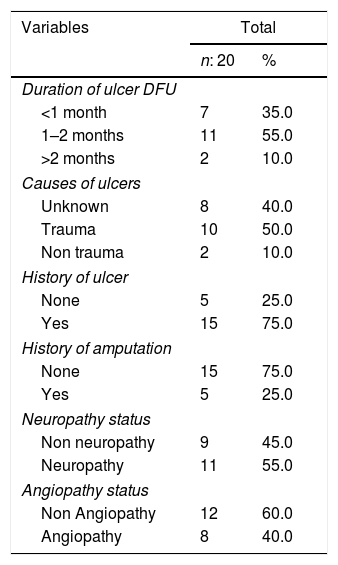
DFU history based on wound duration 1–2 months (n: 11, 55.0%), causes of DFU injuries due to trauma (n: 10, 50.0%), there was a history of previous injuries (n: 15, 75.0%), no amputation history (n: 15, 75.0%), Neuropathy (n: 11, 55.0%), and angiopathy (n: 12, 60.0%) (Table 3).
Frequency distribution of participants based on a history of diabetic foot ulcers and diabetic foot status.
| Variables | Total | |
|---|---|---|
| n: 20 | % | |
| Duration of ulcer DFU | ||
| <1 month | 7 | 35.0 |
| 1–2 months | 11 | 55.0 |
| >2 months | 2 | 10.0 |
| Causes of ulcers | ||
| Unknown | 8 | 40.0 |
| Trauma | 10 | 50.0 |
| Non trauma | 2 | 10.0 |
| History of ulcer | ||
| None | 5 | 25.0 |
| Yes | 15 | 75.0 |
| History of amputation | ||
| None | 15 | 75.0 |
| Yes | 5 | 25.0 |
| Neuropathy status | ||
| Non neuropathy | 9 | 45.0 |
| Neuropathy | 11 | 55.0 |
| Angiopathy status | ||
| Non Angiopathy | 12 | 60.0 |
| Angiopathy | 8 | 40.0 |
The dominant aerobic bacterial colonies found in all participants were Proteus mirabilis (n: 6, 30.0%). Based on The classification of University of Texas Classification system, the dominant type of proteus mirabilis bacteria was found in stage B grade 1 (n: 5, 45.5%), while Eschericia coli bacteria were dominantly found in stage B grade 3 (n: 4, 50%) (Table 4).
Frequency distribution of bacterial types in participants’ diabetic foot ulcer based on the wound classification of University of Texas Classification system.
| The type of bacteria | University of Texas Classification system | |||||||
|---|---|---|---|---|---|---|---|---|
| Stage BGrade 1 | Stage BGrade 2 | Stage BGrade 3 | Total | |||||
| n=11 | % | n=1 | % | n=8 | % | n=20 | % | |
| Alkaligenes faecalis | 1 | 9.1 | 0 | 0 | 0 | 0 | 1 | 5.0 |
| Eschericia coli | 0 | 0 | 0 | 0 | 4 | 50.0 | 4 | 20.0 |
| Enterobacter agglumerans | 1 | 9.1 | 0 | 0 | 0 | 0 | 1 | 5.0 |
| Klebsiella Sp | 1 | 9.1 | 1 | 100 | 0 | 0 | 2 | 10.0 |
| Proteus mirabilis | 5 | 45.5 | 0 | 0 | 1 | 12.5 | 6 | 30.0 |
| Proteus vulgaris | 1 | 9.1 | 0 | 0 | 2 | 25.0 | 3 | 15.0 |
| Providencia alkalifaesen | 1 | 9.1 | 0 | 0 | 0 | 0 | 1 | 5.0 |
| Providencia stuarti | 1 | 9.1 | 0 | 0 | 1 | 12.5 | 2 | 10.0 |
The effect of electrolyzed water in reducing the number of bacterial colonies based on the evaluation time of 2h, 24h, 48h to 72h. This indicates bactericidal effects have occurred at 2h after incubation (Fig. 1).
There are significant differences in the effect of electrolyzed water on bacterial colonization, where ESAW (pH 2.5) significantly (p = 0.001) which confirmed had a better bactericidal effect compared to EWAW, ENAW, EWAlW and ESAlW. However, between ENW pH 7.0 and EWAlW pH 8.5 there seemed no significant difference, where the p value=0.951 (Table 5).
Analysis of the effect of electrolyzed water on bacterial colonization.
| Electrolyzed water | Mean difference | 95% CI | p value |
|---|---|---|---|
| Electrolyzed strong acid water (ESAW) (pH 2.5) | |||
| ESAW (pH 2.5) v.s EWAW (pH 6.0) | −177.4 | (−)204.9–(−)149.8 | 0.001 |
| ESAW (pH 2.5) v.s ENW (pH 7.0) | −253.7 | (−)281.2–(−)226.1 | 0.001 |
| ESAW (pH 2.5) v.s EWAlW (pH 8.5) | −246.8 | (−)274.3–(−)219.2 | 0.001 |
| ESAW (pH 2.5) v.s ESAlW (pH 11.5) | −48.8 | (−)76.3–(−)21.2 | 0.001 |
| Electrolyzed weak acid water (EWAW) (pH 6.0) | |||
| EWAW (pH 6.0) v.s ENW (pH 7.0) | −76.3 | (−)103.8–(−)48.7 | 0.001 |
| EWAW (pH 6.0) v.s EWAlW (pH 8.5) | −69.3 | (−)96.9–(−)41.8 | 0.001 |
| EWAW (pH 6.0) v.s ESAlW (pH 11.5) | 128.7 | 101.1–156.2 | 0.001 |
| Electrolyzed neutral water (ENW) (pH 7.0) | |||
| ENW (pH 7.0) v.s EWAlW (pH 8.5) | 6.9 | (−)20.7–34.5 | 0.951 |
| ENW (pH 7.0) v.s ESAlW (pH 11.5) | 204.9 | 177.3–232.6 | 0.001 |
| Electrolyzed weak alkaline water (EWAlW) (pH 8.5) | |||
| EWAlW (pH 8.5) v.s ESAW (pH 11.5) | 198.0 | 170.4–225.6 | 0.001 |
| Electrolyzed weak acid water (ESAlW) (pH 11.5) | |||
| ESAlW (pH 11.5) v.s ESAW (pH 2.5) | 48.8 | 21.2–76.3 | 0.001 |
| ESAlW (pH 11.5) v.s EWAW (pH 6.0) | −128.7 | (−)156.2–(−)101.1 | 0.001 |
| ESAlW (pH 11.5) v.s ENW (pH 7.0) | −204.9 | (−)232.5–(−)177.3 | 0.001 |
| ESAlW (pH 11.5) v.s EWAlW (pH 8.5) | −198.0 | (−)225.6–(−)170.4 | 0.001 |
Electrolyze water produces strong and weak acid and base solutions.13 PH levels Electrolyzed neutral water is located at 7 to 8. Electrolyzed strong acid water has a pH value of 3 to 2, while electrolyzed strong alkaline water has a pH value of 10 to 13. pH values between 5.0 to 6.5 and 8.0 to 10 each is slightly acidic and slightly alkaline Electrolyzed water has strong bactericidal, fungicidal and virucidal effects in various fields such as medicine.14 Determination of in vitro microbiology whether antibacterial agents are bactericidal or bacteriostatic is influenced by growth conditions, bacterial density, duration of test, and degree of decrease in bacterial count. antibacterial is better described as potentially bactericidal and bacteriostatic.15 In this study ESAW (pH 2.5) had a better bactericidal effect from the first 2h to 72h after the incubation of proteus mirabilis, Eschericia coli, proteus vulgaris, alkaligenes faecalis and enterobacter compared to EWAW (pH 6.0), ENW (pH 7.0), EWAlW (pH 8.5), and ESAlW (pH 11.5). The bactericidal potential possessed by ESAW (pH 2.5) in this study was due to the presence of low pH levels, ORP and residual chlorine. Although EW's bactericidal activity and its mechanism of action are still not fully understood,14 some scientists consider the presence of chlorine in EW as the main factor responsible for bactericidal activity, while others consider ORP to be a major factor.16 Chlorine concentration (Cl2), ORP and pH can affect the bactericidal effectiveness of EW.17 Low pH is known to be responsible for decreasing bacterial production and making bacterial cells more susceptible to dynamic chlorine.19 Bacterial cell inactivation occurs because of a high ORP (1150mV) in acid EW, causing oxidation on the cell surface, damaging various cell layers, and disrupting the metabolic pathway in the cell. In principle, low pH and high ORP in EW Acid acts synergistically with chlorine in reactivating microorganisms.20
The results of this study are in line with previous study which confirmed electrolyzed water acid (pH 2.6) has a disinfecting effect in reducing bacteria,21 thus electrolyzed water acid is effective in reducing bacteria,22 and has a higher bactericidal effect than EW pH 5.6–5.7 and pH 8.23 In addition, the bacterial colonies in EWAW (pH 6.0) is lower than ESAW (pH 2.5) and ESAlW (pH 11.5). Nevertheless, the bactericidal effect possessed by EWAW (pH 6.0) from the first 2h to 72h after the bacteria was incubated is still better than ENW (pH 7.0) and EWAlW (pH 8.5). Active chlorine in the form of hypochlorous acid (HOCl), which dominates when the pH of the solution is 5.0–6.5. HOCl is dissociated with hypochlorite ions (OCl−) at high pH or chlorine gas (Cl2) at low ph.18 This study are in line with previous study that slightly electrolyzed water acid (pH 6.0–6.5) was effective in deactivating Eschericia coli and Staphylococcus aureus.24 One study also reported that slightly acidic electrolyzed water pH range 5.6–5.7 significantly has a bactericidal effect in reducing Eschericia coli.23 Using a slightly acidic electrolyzed water (pH 6.3) has a bactericidal effect in deactivating bacteria25
Electrolyzed neutral water and electrolyzed weak alkaline water in this study have the same lower bactericidal effect on the amount of bacterial colonization compared to other EW types. This is because the decrease in the average number of colonies in these two types of EW is not significant. The optimal pH level for bacterial growth is 4–9.18,26 The ability to deactivate all organisms decreases at pH 9.14
Electrolyzed strong alkaline water (pH 11.5) also has a better bactericidal effect than EWAW (pH 6.0), ENW (pH 7.0), EWAlW (pH 8.5), but is no better than the bactericidal effect of ESAW (pH 2.5) because its bactericidal activity begins at 24h evaluation. The results of this study are consistent with previous finding, that alkaline EW (pH 11.3) has bacterial reduction ability.27
However, with a small sample size and study design, caution must be applied, as the findings might not be different in clinical study, particularly potential negative effect on wound tissue which need more investigation.
ConclusionElectrolyzed strong acid water (pH 2.5) has a better bactericidal effect compared to other types of electrolyzed water. Electrolyzed weak acid water (pH 6.0) has a better bactericidal effect than EW pH 7 and EW pH 8.5 There is no difference in the effect of electrolyzed neutral water (pH 7.0) with electrolyzed weak alkaline water (pH 8.5) on the reduction of bacterial colonization and both have an effect bactericidal which is worse than other EW. Electrolyzed strong alkaline water (pH 11.5) has a better bactericidal effect than EW pH 6.0, EW pH 7.0 and EW pH 8.5.
Conflict of interestThe authors declare no conflict of interest.
Current study granted from Ministry of Research and Technology of the Republic of Indonesia as the magister scholarship.
Peer-review under responsibility of the scientific committee of the International Conference on Women and Societal Perspective on Quality of Life (WOSQUAL-2019). Full-text and the content of it is under responsibility of authors of the article.