Technology Enhanced Medical Education International Conference (THEME 2019)
Más datosDiabetic foot ulcers (DFU) are the main cause of amputations in non-trauma patients. There are risk factors, such as age, diabetic duration, smoking, obesity, hypertension, poor ankle-brachial index (ABI), and high neutrophil/lymphocyte ratio (NLR), for diabetes mellitus patients that can aggravate and are closely related to the occurrence of diabetic foot ulcers. Examining the correlation among these risk factors with the degree of the DFU according to Wagner's classification was the objective of the study.
MethodThis study was a cross-sectional study examining 40 samples of DFU patients at our institution from 2016 to 2020. The results of this study were processed using Spearman's correlation test on SPSS software version 23. Results were considered significant if p-values <0.05.
ResultFrom 40 samples, it was found that duration of diabetes (p=0.018), smoking (p=0.008), ABI (p=0.0001), and NLR (p=0.028) had a significant correlation with degree of DFU according to Wagner's classification. Age (p=0.364), obesity (p=0.87) and hypertension (p=0.73) is not significantly related.
ConclusionThere is a significant correlations between diabetes duration, smoking, ABI, and NLR with the degree of diabetic foot ulcers according to Wagner's classification.
Diabetes mellitus is a chronic metabolic disease characterized by micro- and macro-vascular complications.1,2 With a global prevalence rate of 5%–6%, the International Diabetes Federation (IDF) estimates that the number of diabetics worldwide was 285 million in 2010, and by 2030, the number is expected to be 438 million.2,3
The World Health Organization (WHO) reported that in 2012 more than 347 million people in the world suffer from diabetes mellitus.2,4 The increasing prevalence of diabetes and the hope for longer life causing complications in chronic diabetes has also increased.3
Patients with diabetes mellitus have an increased risk of developing peripheral arterial disease (PAD) in the lower extremities at a rate twice more than those who do not have diabetes. About 15%–25% of patients with diabetes mellitus will develop diabetic foot ulcers during their lifetimes.3,5
Diabetic foot ulcers (DFU) are the main cause of about 85% of amputations in non-trauma patients. This occurs due to extensive infection to gangrene.3,6 Amputation is one of the most feared complications because it affects the health conditions and quality of life of patients.5 The risk of amputation is increased in diabetic patients with peripheral neuropathy in which peripheral neuropathy causes loss of sensation and the ability to recognize problems in the legs, including minor trauma that can easily develop into ulcers.7
Factors related to ulcers in the legs of diabetics are the presence of both neuropathy and angiopathy.8 Diabetic foot ulcers begin with neuropathy and peripheral vascular disorders. Neuropathy and vascular disorders make patients’ feet more susceptible to trauma and infections than non-diabetics’ feet.9,10 Neuropathy is characterized by a sensation of heat, numbness, and a dry feeling.11 Arterial pulsation is still palpable. This condition contrasts with angiopathy or ischemia in which cold palpable legs and palpable pulsation of the arteries are decreased to the point that painless complications, even to the point of necrosis and gangrene, can arise during minor trauma to the area under pressure.12 Some literature have also reported that there are risk factors for diabetic patients that can aggravate and are closely related to the occurrence of ulcers in the diabetic foot such as age, diabetic duration, smoking, obesity, hypertension, poor ankle-brachial index (ABI), and high neutrophil/lymphocyte ratio (NLR).3,7,13–16
Diabetic foot infection and its complications are associated with long wound care duration and an increased risk of surgery and amputations.11,17 Generally, infections begin with small wounds that can develop into infections involving soft tissue, joints, and/or bones if not treated properly.17 In 2008, Abbas et al.18 also reported that the average length of stay in the hospital for diabetic ulcer patients was longer than for non-diabetic patients with ulcers, especially with respect to large ulcers.17,18
As a result of prolonged treatment, the cost of treatment for diabetic foot ulcers will also increase. In the United States, it is estimated that the cost of treatment for an individual DFU is $17.500 while the total cost of treating a diabetic foot ulcer and its complications is $4 billion per year. Harrington et al. reported that total care for patients with DFUs in the United States is $ 1.5 billion per year, while amputations require $150 million.19,20
To be able to handle diabetic foot infections properly, knowledge of the degree of injury is needed to describe the characteristics of the ulcer, such as its location, depth, neuropathy and vasculopathy, infection, and/or the presence or absence of ischemia that can be identified by examining the ABI. At present, several classifications for assessing infected and uninfected diabetic feet have been widely used.19,21
At this time the incidence of diabetic foot is increasing, and patients who come are already in an advanced stage. At presentation, peripheral neuropathy and peripheral ischemia are already in an advanced stage.22,23 The discovery of risk factors causing peripheral neuropathy and early peripheral ischemia is expected to reduce the incidence of DFUs. Examining the correlation among these risk factors with the degree of the DFU according to Wagner's classification was the objective of the study.
MethodsThis study is a cross-sectional study to determine correlations among risk factors: (1) age, (2) diabetic duration, (3) smoking habits, (4) Body Mass Index (BMI), (5) hypertension, (6) ABI, and (7) NLR with the degree of DFU based on Wagner's classification.
In this study, the study population consisted of DFU patients who were hospitalized from January 2016 to April 2020. The number of participants was as many as 40 people who were recruited using purposive sampling technique at Wahidin Sudirohusodo Hospital, Makassar.
Data management included data collection and data tabulation. The collected data were processed using Microsoft Excel 2016 and SPSS 23.0 software (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.) and then were presented in the form of a descriptive table.
To determine the correlation between age, diabetic duration, smoking habit, hypertension, ABI, and NLR to the severity of DFUs according to the Wagner classification. Spearman's correlation test was performed and was found to be significant if the p-value <0.05.
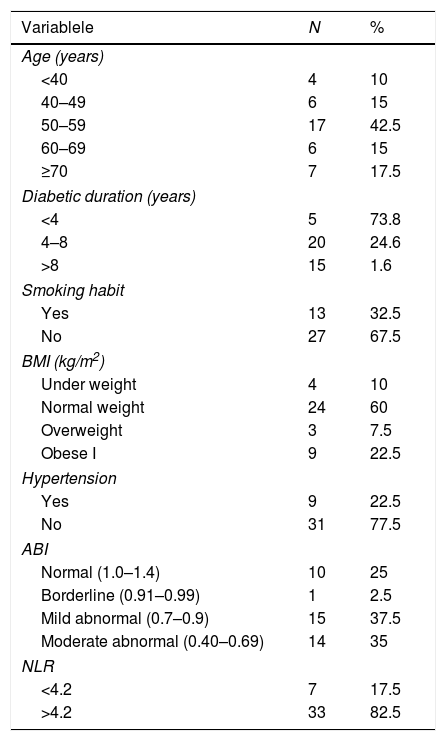
ResultsA total of 40 subjects (Table 1) was recruited who had an average age of 56.38±13 with the oldest and youngest participants of 84 and 33 years, respectively. Diabetic duration between 2 and 35 years with a median value of 6 years, smoking habits as much as 32.5% (13 people) and not smoking as much as 67.5% (27 people). Hypertension was found in 22.5% (nine people) and those with no hypertension was 77.5% (31 people). The ABI had a median value of 0.6. The NLR presented a median value of 8.52.
Characteristics of research participants.
| Variablele | N | % |
|---|---|---|
| Age (years) | ||
| <40 | 4 | 10 |
| 40–49 | 6 | 15 |
| 50–59 | 17 | 42.5 |
| 60–69 | 6 | 15 |
| ≥70 | 7 | 17.5 |
| Diabetic duration (years) | ||
| <4 | 5 | 73.8 |
| 4–8 | 20 | 24.6 |
| >8 | 15 | 1.6 |
| Smoking habit | ||
| Yes | 13 | 32.5 |
| No | 27 | 67.5 |
| BMI (kg/m2) | ||
| Under weight | 4 | 10 |
| Normal weight | 24 | 60 |
| Overweight | 3 | 7.5 |
| Obese I | 9 | 22.5 |
| Hypertension | ||
| Yes | 9 | 22.5 |
| No | 31 | 77.5 |
| ABI | ||
| Normal (1.0–1.4) | 10 | 25 |
| Borderline (0.91–0.99) | 1 | 2.5 |
| Mild abnormal (0.7–0.9) | 15 | 37.5 |
| Moderate abnormal (0.40–0.69) | 14 | 35 |
| NLR | ||
| <4.2 | 7 | 17.5 |
| >4.2 | 33 | 82.5 |
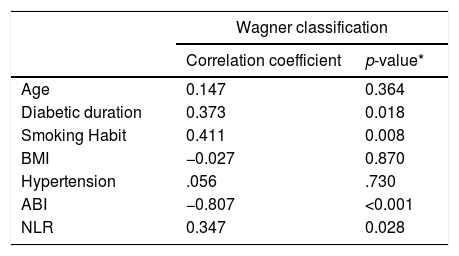
Participants’ age range was 33–84 years with a mean value of 56.38±13 years. The age range of 50–59 years had the highest percentage of participants (42.5%). In this study, there was no correlation found between the degree of DFU according to Wagner's classification and age (p=0.364) as shown in Table 2. Duration of diabetes ranged from 2 to 35 years with a median value of 6 years. A significant correlation was obtained between DFU degrees according to Wagner's classification and diabetes duration (p=0.018) with a correlation coefficient of 0.37, which means the correlation between the two variables was weak, whereas the higher the Wagner's degree, the longer the duration of diabetes mellitus was (Table 2). Smoking habits were found in 32.5% (13 people) and 67.5% (27 people) were non-smokers. A significant correlation was obtained between the degree of DFU according to Wagner's classification and smoking habits (p=0.008) with a correlation coefficient of 0.41, which indicates the higher the Wagner degree, the more smoking habits occur (Table 2). There is no relationship between the degree of DFU according to Wagner's classification and body mass index (p-value=0.87) as shown in Table 2. A history of hypertension was found in 22.5% (nine people), and 77.5% (31 people) had no hypertension. In this study, no correlation between DFU degree according to Wagner's classification and hypertension (p-value=0.73) was found (Table 2). The ABI value had a median value of 0.6, and a significant correlation was obtained between the ABI value and the DFU degree according to Wagner's classification (p-value=<0.001) with a correlation coefficient of −0.807, which indicates the correlation between the two variables was very strong, whereas the lower the ABI value was, Wagner's degree increased (Table 2). The value of the NLR in this study was determined to be low when it was <4.2. The median value of NLR was 8.52. A significant correlation was obtained between DFU degrees according to Wagner's classification and NLR (p-value=0.028) with a correlation coefficient of 0.34, which indicates the correlation between the two variables was weak, whereas a higher Wagner's degree, the higher the NLR value was (Table 2).
Correlation between between age, diabetic duration, smoking habit, BMI, hypertension, ABI, and NLR to the Wagner classification.
| Wagner classification | ||
|---|---|---|
| Correlation coefficient | p-value* | |
| Age | 0.147 | 0.364 |
| Diabetic duration | 0.373 | 0.018 |
| Smoking Habit | 0.411 | 0.008 |
| BMI | −0.027 | 0.870 |
| Hypertension | .056 | .730 |
| ABI | −0.807 | <0.001 |
| NLR | 0.347 | 0.028 |
Although age was not related with the degree of DFU statistically, in this study, we still concluded that these two parameters were related as we can see the mean value is 56.38±13 years. Based on a study by Jeyaraman et al.,24 it was concluded that there was a correlation between age (with a median age of 64 years) and the occurrence of DFUs. Increased age was shown to cause an increased risk for angiopathy. People who were >40 years appear to be at risk of developing angiopathy.24,25 Jeffcoate et al.26 reported that in older people, wound healing in patients with DFU was more difficult. This finding might have been caused by a worsening of vascular function as people age so that in old age, infection happen at a higher rate than at young ages.27,28
In a study by Al-Rubean et al.,29 a significant relationship between diabetes duration and the occurrence of DFU was found in which 88.99% of the study subjects had diabetes mellitus for more than 10 years, and smokers tended to be 1.15 times more likely to develop gangrene, which increased the incidence of amputation. Ledoux et al.,30 put forward the obesity paradox theory in which for every 5kg/m2 increase in body mass index (BMI), a reduced risk of DFU was found. Sohn et al.,31 reported a low risk of DFU among overweight and obesity patients with a BMI of 25–34.9. Biasucci et al.,32 reported wound healing in obese patients was better because there was an increase endothelial progenitor cell levels that function as a protective vascular factor against atherosclerosis.
Hypertension was found in 22.5% (9 people), and 77.5% (31 people) had no hypertension. No correlation between DFU degree according to Wagner's classification and hypertension (p-value=0.73) was found. This finding may have happened because in the nine patients with a history of hypertension, seven of them underwent routine antihypertensive treatment. Abouhamda et al.,15 stated that an ABI value of <0.9 is an independent risk factor for amputation of inferior locomotion in those suffering from DFUs. Taufik et al.,33 found a correlation between the ABI value and the degree of severity of the DFU according to Wagner's classification. Vatankhah et al., found that the increase in the value of the NLR was directly proportional to the worsening of wound healing in patients with DFUs. Altay et al.,34 stated that patients with high NLR values have a higher degree of DFU, and the need for vascular treatment and amputation is much higher than patients with low NLR values.
ConclusionThere is a correlation between the degree of DFU according to Wagner's classification with diabetic duration, smoking habits, ABI, and NLR. No correlations between the degree of DFU according to Wagner's classification with age, BMI, and hypertension were found.
Ethical approvalAll procedure for this study has been approved by Ethics Commission Faculty of Medicine, Hasanuddin University Number: 325/UN4.6.4.5.31/PP36/2020.
ConsentThe research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The patients have given their written informed consent on admission to use their prospective data base and files for research work.
FundingThe authors declared that this study has received no financial support.
Author contributionDS, MW, Pri, JH, NM, SM, JR and MF wrote the manuscript and participated in the study design. DS, MW, NM, Pri, and MF drafted and revised the manuscript. DS, MW, and JR performed the treatment and surgery. JH, MF, and Pri performed bioinformatics analyses and revised the manuscript. All authors read and approved the final manuscript.
Conflict of interestThe authors declare no conflict of interest.
Peer-review under responsibility of the scientific committee of the Technology Enhanced Medical Education International Conference (THEME 2019). Full-text and the content of it is under responsibility of authors of the article.