Technology Enhanced Medical Education International Conference (THEME 2019)
Más datosHigh Grade Astrocytoma (HGAs) is a type of Glioma tumor, observed in less than 15% of all primary neoplasms in the central nervous system, and brain abscess is a relatively rare infection of the parenchyma area. This report is based on HGA cases resembling brain abcesses in girls with symptoms of progressive severe headache accompanied with hemiplegia.
Case presentationAn 11-year-old girl was admitted with symptoms of headaches experienced over a period of 4 months. The pain was more progressive, and the patient also has left hemiplegia. Furthermore, head CT scan results with contrast and brain MRI showed a brain abscess (space-occupying lesions – SOL), and tumor removal surgery was performed. The HGA results of Anatomy Pathology were obtained, and therapy was continued with the administration of the HIT-GBM-C protocol. Therefore, a follow-up was performed at 3, 6, 12, and 24 months, and no signs of recurrence were observed. Also, on the outcome of physical examination and brain MRI was within the normal limit.
ConclusionThis report shows a rare case of HGA evaluated to share similarities with the incidence of cerebral abscess in children. The tumor diagnosis is based on clinical, radiological and histological examination, principally handled through multi-modality therapy.
Brain tumors are extremely rare in the first decade of life, with an incidence of 1 per 100,000 babies. However, the probability increases with age, and a range of 2–5 cases per 100,000 is observed amongst 2 year old children.1 In addition, primary brain tumor cancer is the leading cause of death in youngsters and adolescents <20, and third foremost in adults aged 20–39, thus exceeding leukemia.2 However, the prevalent type recognized in children with primary malignant central nervous system (CNS) tumors include solid organ growth, comprising 20% in ages 1–4 and 30% in 5–9 years.3 Moreover, brain abscess is a relatively rare intracerebral infection initiated at a localized part of the brain, which often develops into a collection of pus, surrounded by well-vascularized capsules.4 However, about 25% of all types occur in individuals aged less than 15 years old, with a peak incidence of the age of 4–7.5
This study focused on cases of HGAs with close similarity to brain abscess, in girls with symptoms of progressive severe headaches, accompanied by hemiplegia.
Case reportAn 11-year-old girl with 4-month progressive headache was admitted to an outpatient unit with complaints of blurred eyesight and worsened weakness in the left limb, causing the inability to walk. The alternative treatments previously adopted include herbal medicine and acupuncture, before a tertiary hospital diagnosed and incompletely treated cerebral abscess for 8 days in August 2018. Furthermore, history of weight loss, seizures, similar family illness and cancers were denied. However, previous visit to a neurologist recommended Magnetic Resonance Imaging (MRI) check, and the result showed cerebral abscess.
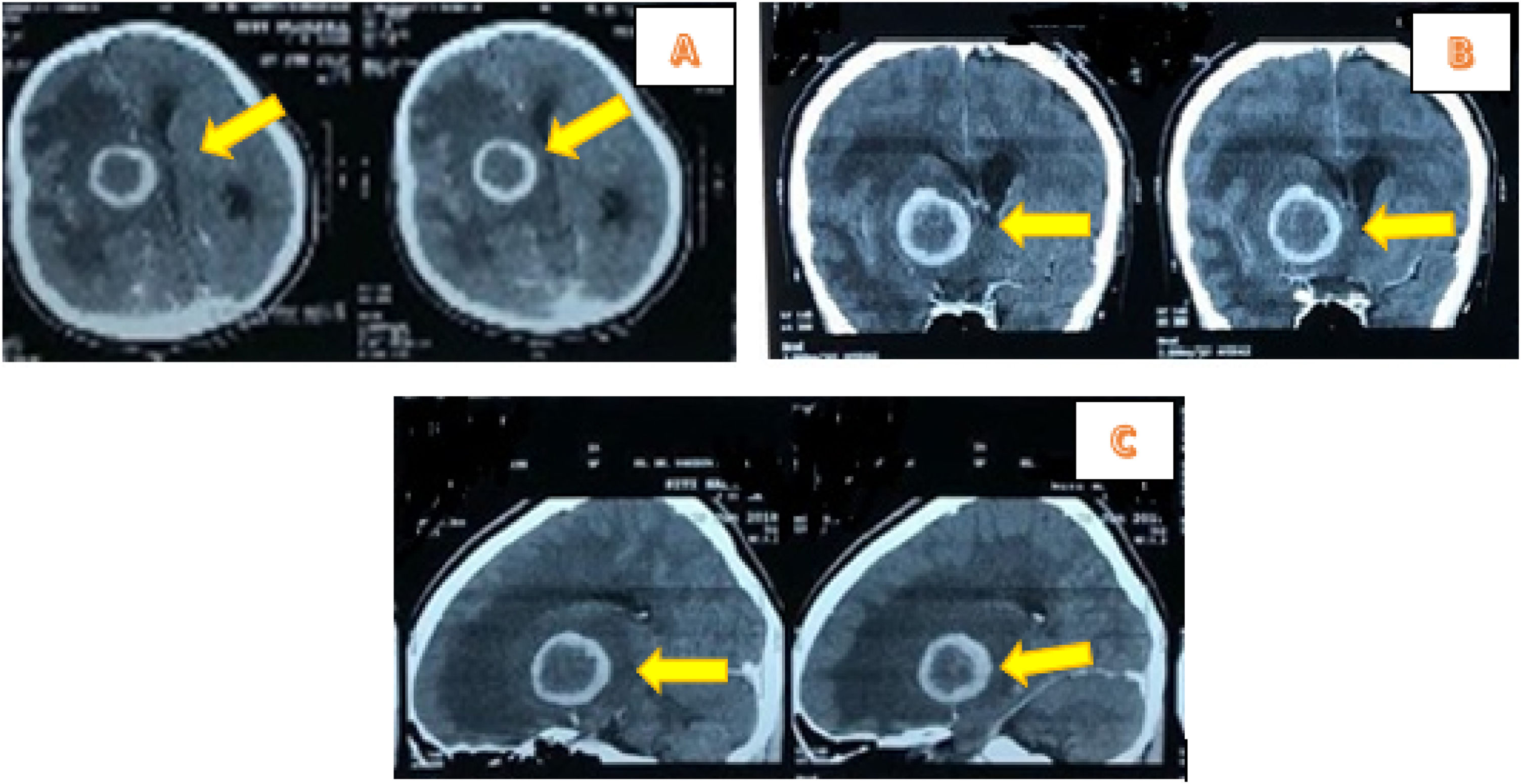
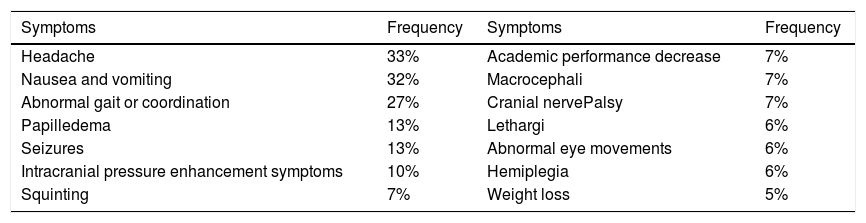
The vital signs examination showed normal limits. Glasgow Coma Scale (GCS) consciousness status was E4V5M6, while neurological status in upper left extremities was 4 and lower left extremities was 3. The examination of the left eye vision obtained 1/∼ and Contrast Head CT Scan indicated hypodense lesions with hyperdense rim and firmly irregular borderline size 3.8cm×3.7cm×3.6cm. The right frontotemporal contrast showed enhanced rim accompanied by extensive perifocal edema, causing herniation subfalcine of 1.6cm to the left, and contrast lesions enhanced pons size 2cm×2.2cm×1.9cm of abscess accompanied by obstructive hydrocephalus marks (Fig. 1).
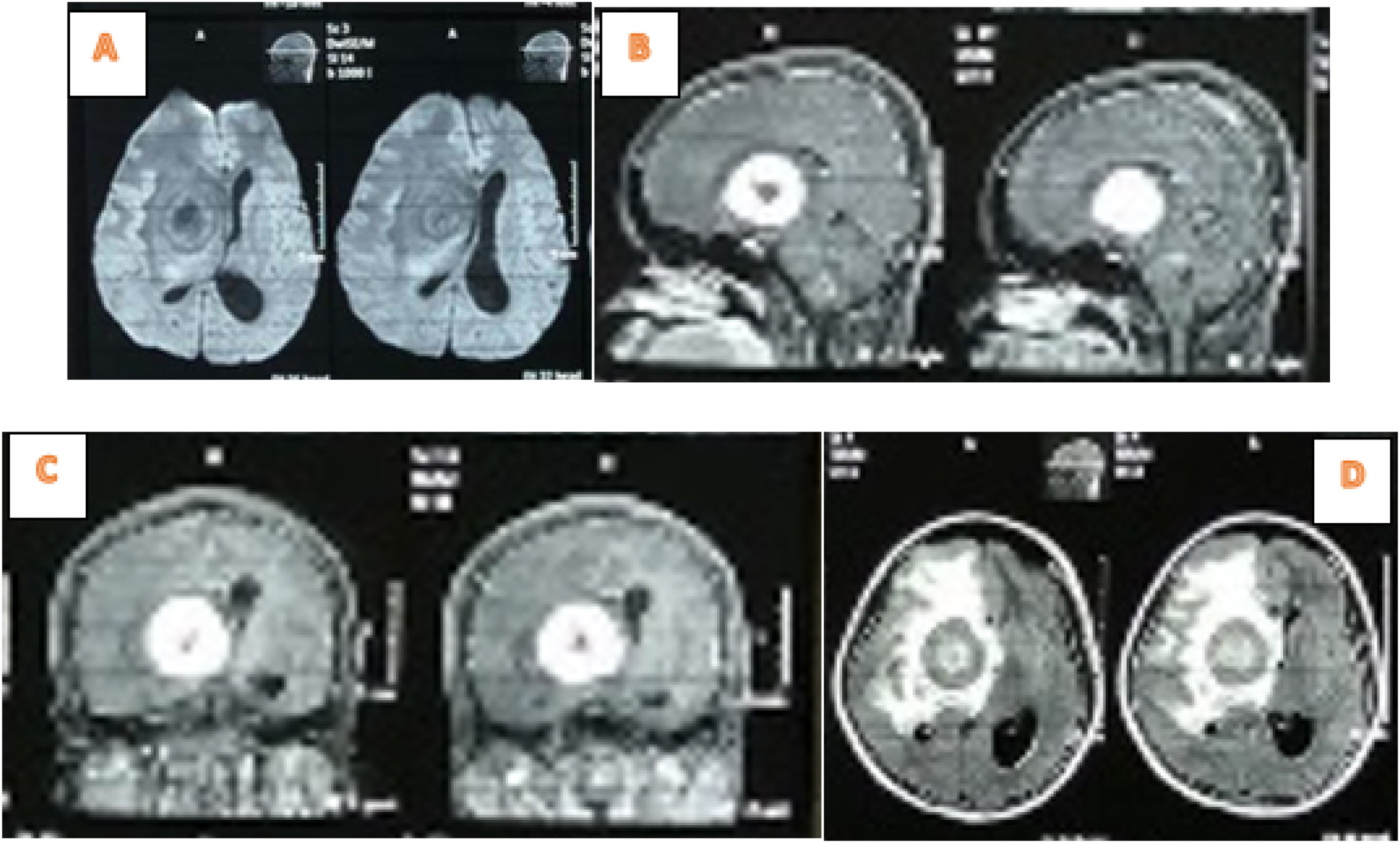
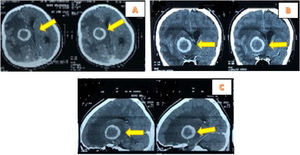
The brain MRI examination showed thick irregular rim-enhanced wall lesion of 4.6cm×4cm×4.6cm in the right frontotemporal, with perifocal edema. This was implicated in the onset of subfalcine herniation to the left 1.3cm; Rim enhanced lesions with pons indistinct boundary 1cm×1cm and right cerebellar peduncle with size 1.4cm×1.2cm. The right frontotemporal abscess impression-pons and right cerebellar peduncle was accompanied by signs of obstructive hydrocephalus (Fig. 2).
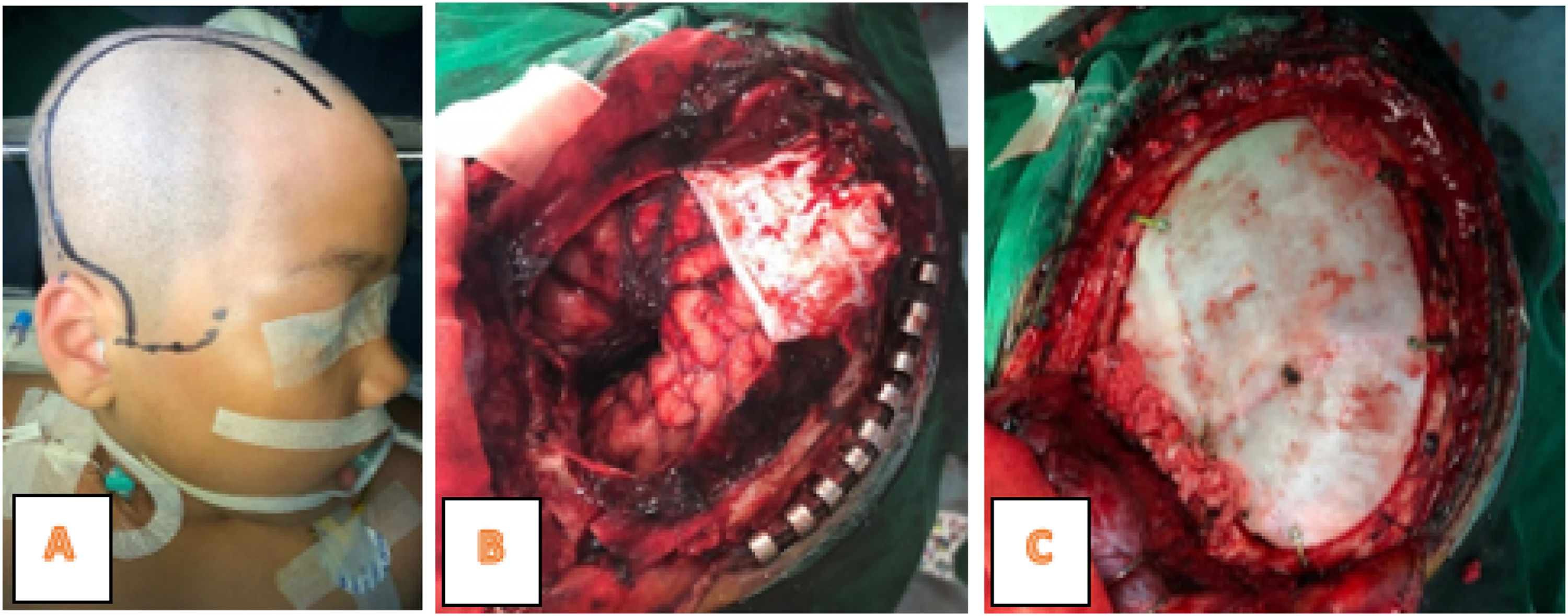
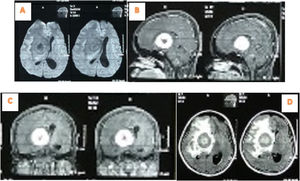
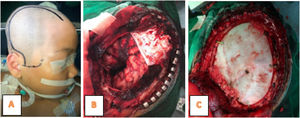
The laboratory tests showed Hb was 12.4g/dL, leukocytes 11,500mm3, platelets 417,000/mm3, Neutrophils 63%, and therefore a diagnosis of Intracranial SOL and a tumor removal operation was carried out. Furthermore, tumor mass was found in the right frontotemporal at the surgery time (Fig. 3), hence extensive resection and anatomic pathology examination were performed.
Anatomical pathology examination results of a malignant astrocytoma (High Grade Astrocytoma. grade III) (according to the 2016 CNS WHO) (Fig. 4).
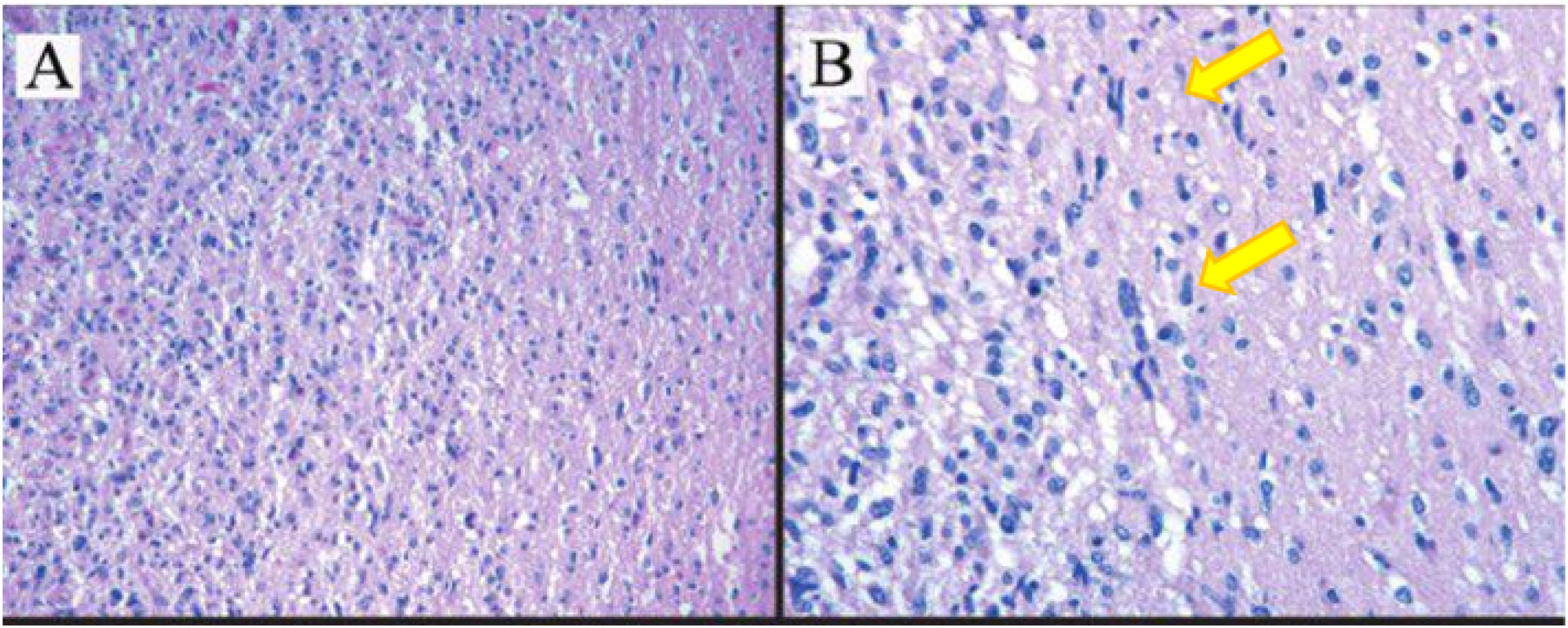
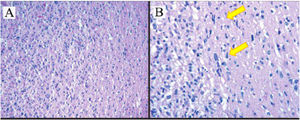
Histopathology results of astrocytoma tumors show tumor cells diffusive infiltration into the brain parenchyma with atypical nucleus, pleomorphic, coarse chromatin and partially with prominent nucleoli. (A) Hematoxylin–eosin staining, 10×. (B) Hematoxylin–eosin staining 100× (yellow arrow showed astrocyte cells with bizarre nuclei).
The patient was treated in intensive care for 8 days and discharged after 10 days in good condition. This was followed by chemotherapy in the oncology division with HIT-gBM-c regiment, comprising cisplatin, etoposide, vincristine, and ifosfamide was administered with conventionally fractionated local radiotherapy of 54 or 59.6Gy, while Valproic acid served as maintenance treatment regimen for further management. In addition, a total of seven cycles of HIT-gBM-c regimen was received, and follow-up was performed at 3, 6, 12, and 24 months. This showed no recurrence signs on physical examination, while brain MRI was within normal limits.
DiscussionThis report is based on high grade astrocytoma in children initially alleged to have a brain abscess (SOL), characterized by symptoms of chronic progressive headache and hemiplegia. In addition, the head CT scan and MRI results showed a brain abscess different from the anatomic pathology examination, indicating high grade astrocytoma.
HGAs in children occur in less than 15% of all primary central nervous system neoplasms, including tumors located in the supratentorial region. This comprises the cortex and thalamus, HGAs in children similar to adults, observed in the brain stem. However, there is an exclusive location for these tumors in children, termed the diffuse intrinsic pontine gliomas (DIPGs).6
Based on the World Health Organization (WHO) classification system, 75% of primary CNS tumors in children originate from neuroepithelial (gliomas).3 These include embryonal, neuronal CNS astrocytomas and a mixture of neuronal-gliomas and ependimomas, and are most often recognized in children and adults.7 According to the WHO gliomas are classified into 4 main groups, comprising astrocytomas; oligodendrogliomas; mixed oligoastrocytomas; and ependymal tumors.8,9 These are further divided by histological differences into WHO grade I to IV, and based on cytology and histology into cellularity, mitotic activity, atypical nucleus, microvascular proliferation and necrosis.9
Brain abscess (BA) is a focal central nervous system infection involving the cerebral parenchyma. This condition is rare in adult populations, and pediatrics.10
In the United States, over 3700 new cases of CNS tumors are diagnosed annually in children aged <19, with an approximate total case of 26,000, and 5-year survival rate in 66%.3 The average incidence was higher in males than females (29.9 vs. 25.1 per million) and prevalent in Caucasian races (29.4 per million).11
About 25% of all brain abscesses occur in children <15 years old, and peaks at the age of 4–7.10,12 The most common predisposing factors are cyanotic congenital heart disease (hematogenous spread), and a direct flow from nearby locations (middle ear, sinus, teeth).12 In addition, about half of the brain abscesses occur because of sinusitis, otitis, or dental infections.13 The patient in this case study lacked any predisposing factors, as physical examination of the ear and teeth area was within the normal limit. Also, the abscess location, based on imaging was not closely related to the infection source, including the presence of frontal or etmoidal sinusitis.
Supratentorial tumors occur in 31% of children with CNS tumors.3 This phenomenon is more common in individuals less than 1 year old, and older children, with the higher tendency to develop infratentoria tumors, and adults, with predominant regional tumors.11 The important factors considered in brain tumors diagnosis are location, age and cell type. Moreover, tentorium divides the brain into two compartments, where the above (supratentorial) comprise of cerebral hemispheres, basal ganglia and thalamus. However, the lower region (infratentorial) consists of pineal gland, tectum, pons, medulla and cerebellum.14
The predisposing conditions and location of the abscess provide clues related to possible etiology. In addition, brain abscesses associated with the direct spread of sinus or the odontogenic focus is predominantly in the frontal region. These are triggered by aerobic or anaerobic streptococci, including Streptococcus milleri, Staphylococcus aureus and Enterobacteriaceae. However, abscesses secondary to ear infections are usually temporal or cerebellar, characterized by a mixed flora etiology, comprising Pseudomonas aeruginosa, Enterobacteriaceae and Streptococcus. In addition, Streptococci, S. aureus, and Enterobacteriaceae have been implicated in the incidence of post-traumatic abscess.12 The multiple monomicrobial forms are implicated by the hematogenous spread of distant focus in the distribution of the cerebral arteries media. This condition is common in neonates and patients with immune disorders.12,15
Therefore, high-grade astrocytomas found in patients were supratentorial and infratentorial (in the right frontotemporal – pons and right cerebellar peduncle), and this is evenly divided in children. In addition, the pediatric population distribution depends on age, with a predominance of supratentorial in children under 2 and infratentorial in 3–15 years.14
Furthermore, based on cytology, the histological differences is divided into WHO grade I to grade IV, and includes cellularity, mitotic activity, atypical nucleus, microvascular proliferation and necrosis.9 The important prognostic factors comprise tumor grade, age of patient at first diagnosis, and total resection without any residual tumor. However, glioblastoma multiforme (GBM, grade IV astrocytoma) is incurable despite extensive therapeutic efforts for decades.16
In addition, age influences the tumor location, grade, pathological subtypes and the potential for gradual development from low to high category. Hence, grade I astrocytomas called pilocytic astrocytomas (PAs) are dominant and accounts for 25% of all child brain tumors.7,9
The clinical presentation of brain abscesses is influenced by several factors, including pathogen virulence, lesion location and host immune status. In addition, fever, headache and vomiting occur in 60–70%, while changes in mental status, incidence of seizures, and focal neurological signs ensue in 25–50%. The classic triad of focal deficits, headaches, and fever have been reported in <30%, and ≤25% have meningeal signs.12
The signs and symptoms of pediatric brain tumors vary based on the malignancy type, location and age,14 and are seen for 6 or more months in over 50% of patients (Table 1).3 However, in situations without seizures or focal neurological deficits (including diplopia caused by sixth nerve paresis), majority of symptoms are non-specific and less serious. Therefore, common indications comprise of headaches, nausea, vomiting, lethargy, changes in personality, and deterioration in school performance.14 These patients are aged 2–14, and usually have prolonged headaches, nausea or vomiting, ataxia, and diplopia.11,14 The cause is hydrocephalus and due to ventricular obstruction, usually located in the midline posterior fossa.14
The most common symptom experienced by patients with supratentorial brain tumors in children.3
| Symptoms | Frequency | Symptoms | Frequency |
|---|---|---|---|
| Headache | 33% | Academic performance decrease | 7% |
| Nausea and vomiting | 32% | Macrocephali | 7% |
| Abnormal gait or coordination | 27% | Cranial nervePalsy | 7% |
| Papilledema | 13% | Lethargi | 6% |
| Seizures | 13% | Abnormal eye movements | 6% |
| Intracranial pressure enhancement symptoms | 10% | Hemiplegia | 6% |
| Squinting | 7% | Weight loss | 5% |
Also, two factors lead to the nausea and vomiting, comprising increase in intracranial pressure (ICP) and direct irritation/infiltration of the vomiting center (postrema area). The postrema is located at the fourth ventricle base, and is vulnerable to malignancies from large posterior fossa compression or direct brain stem invasion.14,17 The frequent visual disturbances associated with posterior fossa tumors includes diplopia, difficulty in finding (sunsetting eye or Parinaud's syndrome), and occassional decreased visual acuity, due to papilledema. Furthermore, blindness is a common symptom of supratentorial tumors because of the optic nerve atrophy from direct compression.3,14 The patient in this case has no symptoms of fever and lateralization. There was a predominant complaint of headaches accompanied by visual disturbances as a result of increased ICP. Furthermore, MRI examination with contrast is needed to distinguish cerebral abscesses and HGA. Particularly, abscesses comprise of smoother inner walls, and satellite lesions support infection,18 However, there is a possibility of low intensity capsules,18,19 relative brain blood volume (rCBV), increased high-grade gliomas and reduced abscesses; conversely, cystic components tend to not show limited diffusion.18
MRI of the spinal cord is necessary for tumor staging in all cases. Also, immediate hydrocephalus management is frequently required in children with severe symptoms, while CS temporary transfer through external ventriculostomy or shunts is performed before tumor removal.11 In this case, as many as possible tumors were removed (gross total resection) and chemotherapy and radiotherapy was continued with HIT-gBM-c regimen. GTR (Gross Total Resection) surgery is performed, being the best predictor of survival rates for pediatric patients with glioblastoma,20 and significantly related to OS (overall survivor) except where the growth is in the brain stem.1,3,11,14
Therefore, the surgical intervention goal is to safely remove majority of tumor tissue to obtain a histological diagnosis, and an uninterrupted cerebrospinal fluid (CSF) lane. The tumor location often determines the aggressiveness of discharge, since prognosis is indicated by the remaining postoperative tumors.3,14
In addition, child HGG survival rate is higher than adults, with 5-years-old at 19%. This is achievable with intensive chemotherapy protocols or, the influence of a biologically different HGGs in children.22 However, prognosis is worse in younger children, estimably due to radiotherapy delays, and multidisciplinary doctors are to perform patient's follow-up with HGG.23
However, in performing the tumor excision surgery, a neurological deficit usually arises in 30% of postoperative patients, due to proximity in the brain stem and enlarged and soft cranial nerves (edema), but half of this is transient.1 The occurrence of postoperative deficits often results from tumor proximity to the brain stem and cranial nerves. This is predominantly characterized by enlarged and soft (edema), although the care provided in the intensive care room attenuated the incidence of any neurological deficit.
Chemotherapy agents including temozolomide, bevacizumab, cisplatin, etoposide, vincristine, and ifosfamide are frequently used to treat children.11 Furthermore, patient survival rates increase significantly where HGG is performed by GTR, and also the HIT-gBM-c protocol (intensive vincristine-cisplatin chemotherapy, ifosfamide and etoposide before as well as after radiotherapy followed by oral valproic acid provision as maintenance therapy).11,21,23
The important prognostic factors for astrocytoma include tumor grade, patient age on first diagnosis, and total resection, without any residue.16 In addition, child with HGG survival rate is higher than adults, with 5-years-old at 19%.20,24 This is achievable with intensive chemotherapy protocols or, the influence of a biologically different HGGs in children.24 However, prognosis is worse in younger children, estimably due to radiotherapy delays, and multidisciplinary doctors are to perform patient's follow-up with HGG.23,25
ConclusionThis report is focused on a rare case of HGA, characterized by some similarities with cerebral abscess. The occurrence in children is in the form of a rare tumor in the central nervous system. In addition, diagnosis is based on clinical, radiological and histological examination, while the disease management requires multi-modality therapy.
Authors’ contributionsDR, NAL, AAI, DW, WA, AIH, and MF researched the literature and wrote the manuscript. DR and WA operated on the patient and had the idea for this case report. DR, NAL, AAI, DW, and WA checked the manuscript and made corrections. AT, NAL, AIH, and MF provided the overall guidance and support. All authors read and approved the final manuscript.
Ethics approval and consent to participateOur institution does not require ethical approval for this case report.
Consent for publicationWritten informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Availability of data and materialNot applicable.
Conflict of interestThe authors declare no conflict of interest.
FundingNot applicable.
Peer-review under responsibility of the scientific committee of the Technology Enhanced Medical Education International Conference (THEME 2019). Full-text and the content of it is under responsibility of authors of the article.