Technology Enhanced Medical Education International Conference (THEME 2019)
Más datosVascular endothelial growth factor (VEGF) is a trophic factor that is expressed in the central nervous system after brain injury. It is expressed in the brain after traumatic brain injury and functions as a powerful activator of angiogenesis and vascularity. Furthermore, VEGF is mitogenic and a potential mediator of vascular permeability. Various biochemical mediators of vascular permeability have been studied, but there is no specific therapy aimed at modulating cerebrovascular permeability that has been used as a clinical standard. We examined the effects of vascular permeability through VEGF expression in blood vessels after head trauma to Sprague-Dawley rats. We measured levels of VEGF in groups of rats that were given Caffeic Acid Phenethyl Ester (CAPE) and those that were not.
MethodsWe examined the effects of CAPE in 2 groups of rats subjected to brains injury. VEGF levels in blood were measured 3 times: 24h before trauma and on days 4 and 7. The parameters of the serum VEGF levels were examined with sandwich ELISA.
ResultsWe found a significant decrease in VEGF levels (p<0.05) in the group that was given CAPE than in the group that was not on day 4 and day 7.
ConclusionThe group that was given CAPE had lower VEGF levels in rat a model of brain injury. VEGF is an indicator of vascular permeability activity in the blood, and CAPE can decrease it.
Brain injury is a serious problem for developing countries and developed countries. Low public awareness among drivers is also a major problem that causes high incidence of brain injury. The incidence of brain injury is very high among those under 45 years old, and there are approximately 52,000 cases of deaths and 80,000 cases of severe permanent neurological disability due to brain injury.1 Traumatic brain injury triggers various pathological processes that occur in the brain shortly after trauma. This pathological process can occur directly (direct mechanical disruption of brain tissue, or primary injury) and indirectly (secondary injury).2 This secondary mechanism induces an acute inflammatory response, including blood brain barrier injury, brain edema, peripheral blood cell infiltration and activation of immunocompetent cells, and intrathecal release from many inflammatory mediators, such as interleukins and chemotactic factors.2,3
In the last two decades, much research has been carried out in the search for drugs that can help to prevent the pathological process and to improve the pathological process once it has already started.4 Various mediators involved in either establishing or aggravating this process have been identified,5 including vascular endothelial growth factor (VEGF), arachidonic acid, bradykinin, Ca2+, glutamate, and free radical oxygen.2,5,6 Modulation of some mediators was found to be useful in experimental studies, but there were no beneficial clinical effects or unseen side effects.5,7
VEGF is also known as vascular permeability factor because of its ability to induce vascular leakage.4,8 There is a negative response from the increase of VEGF in the form of increased of cerebrovascular permeability, which results in brain edema.9,10 In addition, increased endogenous VEGF can interact with its receptors in ischemic vessels and contribute to blood brain barrier damage and subsequent leakage.8,10,11 Although the relations between increased endothelial cell permeability, mitogenic properties, and angiogenic properties is uncertain, VEGF and its endothelial cell receptors are expressed in non-neoplastic pathology processes, including hyperpermeability to plasma protein circulation, retinopathy, rheumatoid arthritis, psoriasis, and other inflammatory conditions.8,11,12
Some antioxidants have been shown to provide neurovascular protection after stroke in experimental animals,7 such as some natural flavonoids found in fruits and vegetables.13 Caffeic Acid Phenethyl Ester (CAPE) is a part of the flavonoid group, and some of its features are that it can be absorbed quickly and metabolized by plasmatic esterase,14 it is non-toxic to humans, and it is available as an active component of propolis from honey bees.15 In addition to NF-KB inhibition,16 CAPE also inhibits lipoxygenase,17 protein tyrosine kinase,18 and lipid peroxidation.19 In this study, we examined the effects of vascular permeability through VEGF expression in blood vessels after head trauma in Sprague-Dawley rats. We measured levels of VEGF in groups of rats that consumed CAPE and those that did not.
Materials and methodsCAPE was purchased from Sigma-Aldrich Pte. Ltd. with Reagan number 10454-70-9. It was diluted in saline solution and given to rats by intraperitoneal (IP) injection of 10mg/kg body weight (BW) every day for 7 days. The control group was given a placebo. IP CAPE injection was carried out to ensure that a certain amount of CAPE would enter the gastrointestinal cavity.
AnimalsA total of 10 Sprague-Dawley male rats weighing 200–300g were used in this study. rats were divided into 2 groups: a CAPE treatment group and a group without CAPE treatment. All animal procedures have been approved by our local Ethics Committee (number: 771/UN4.6.4.5.31/PP36/2019).
Surgical procedureThe surgical procedure was performed using aseptic techniques. The 10 male Sprague-Dawley rats were free from viruses and other pathogens and more than 2 months old. They weighed 200–300g and had unlimited access to food and drink until the time of the study. The rats were placed in two groups: (1) head trauma with CAPE treatment and (2) head traumas without CAPE treatment. The anesthetic was 1mg/kg of diluted ketamine, and head trauma was applied with the modified Marmourou model.20 Anesthetized rats were cleaned using povidone-iodine, and coronal incisions were made through the midline of the skull. A bore hole was made with a high-speed dental drill with care to expose injection dura mater.
Craniectomy was carried out along 1.5cm, and a mass of 20g was dropped from a height of 20cm by passing through a tube. The rats’ heads with exposed dura mater were placed just below the tube, and the mass of 20g21 hit the head. To ensure the trauma model resulted in damage to the brain, 1 rat was sacrificed for pathology examination with hematoxylin staining to confirm the presence of bleeding in the brain tissue.
The other rats were treated according to craniotomy procedures. We sutured the incision wound with non-absorbable 5.0 suture (silk) and treated it with topical antibiotics. All surgical procedures were performed aseptically by adhering to the principle of sterility. After the surgery and trauma, all animals were kept at room temperature for recovery and returned to their cages according to their groups.
VEGF sample examinationVEGF sample examination was carried out by collecting blood 3 times: on day 0 (24h) before the trauma and surgery and then on day 4 and day 7 after treatment. All blood samples were examined by VEGF Sandwich ELISA with Catalog No. Ls-F978 from Life Span BioSciences, Inc.
Statistical analysisThe data are presented as the mean±standard deviation (SD). All data were computed and analyzed using Excel 2013 and SPSS Statistics 23.0 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.). VEGF was tested with an independent t-test. p values less than 0.05 were considered statistically significant.
ResultsAll rats survived after trauma until the predetermined experimental time was reached.
The characteristics of the animal models are listed in Table 1. The rats’ weights were not significantly different (Lavene homogeneity test, p value >0.05).
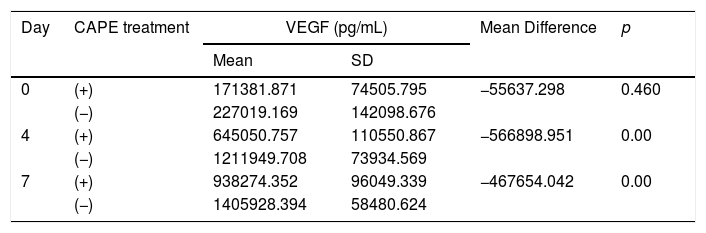
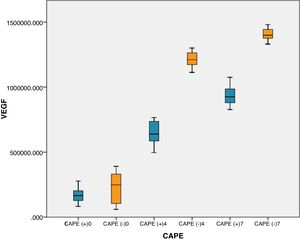
On days 4 and 7, rats with brain injury that were given CAPE had lower mean VEGF levels compared to groups without CAPE treatment. There were significant differences in VEGF levels in rats with CAPE treatment and without CAPE treatment (p<0.05). On day 7, VEGF levels with and without CAPE treatment were higher than on day 4 (Table 2 This can also be seen clearly from the boxplot below (Fig. 1).
Effects of CAPE administration on VEGF levels in rats with brain injury.
| Day | CAPE treatment | VEGF (pg/mL) | Mean Difference | p | |
|---|---|---|---|---|---|
| Mean | SD | ||||
| 0 | (+) | 171381.871 | 74505.795 | −55637.298 | 0.460 |
| (−) | 227019.169 | 142098.676 | |||
| 4 | (+) | 645050.757 | 110550.867 | −566898.951 | 0.00 |
| (−) | 1211949.708 | 73934.569 | |||
| 7 | (+) | 938274.352 | 96049.339 | −467654.042 | 0.00 |
| (−) | 1405928.394 | 58480.624 | |||
Independent t-test with p value <0.05 is statistically significant; SD=standard deviation.
Besides being mitogenic, angiogenic, and a potential mediator of vascular permeability, VEGF also functions as a powerful angiogenesis and neurogenesis activator. In addition, VEGF also correlates with tumors and their development.22 CAPE's anti-tumor activity has been investigated to reveal its effect on colon adenocarcinoma cells (CT26).23 However, the effectiveness of CAPE on brain neoplasms related to VEGF has not been widely reported compared to the use of steroids with brain tumors associated with VEGF.24 The antagonists role of VEGF in reducing brain edema has also been reported through the administration of mFlt(1-3)-immunoglobulin G in mice evaluated with high-resolution MRI, which showed a significant decrease in edema volume one day after ischemic onset.10
This study showed a significant decrease in VEGF after CAPE treatment on day 4 and day 7 when compared to the group without CAPE treatment. This shows the effectiveness of CAPE administration on the vasogenic process of overcoming brain edema. VEGF activity increases in the edema process associated with neoplasia, which is due to the role of VEGF as a mediator associated with microvascular tumors and vascular permeability.10
VEGF is a trophic factor that is expressed in the central nervous system after injury11 and induces angiogenesis.25 VEGF can also have beneficial effect on the survival of newly formed nerve precursors26 and has been implicated in neurogenesis after cerebral ischemia27 and neurite development.28 Furthermore, VEGF has important implications in increasing the size of the subventricular zone (SVZ),29 and inhibition of VEGF expression after injury might worsen the outcome.30 However, VEGF can be a double-edged sword because it can increase the permeability of blood vessels and worsen cerebral edema, causing undesirable effects.31 Furthermore, as shown in previous studies, VEGF antagonists can reduce the formation of brain edema.10
VEGF is a vascular permeability factor that functions as a regulator of angiogenesis and vascular permeability.32 These molecules bind to endothelial cells through interactions with high-affinity tyrosine kinase receptors flt-1 (VEGFR-1) and Flk-1/KDR (VEGFR-2). These receptors are predominantly produced in SDO endothelial cells.33 VEGF has very strong vascular permeability activity (several thousand times stronger than histamine) and has a direct effect on tight junction SDO endothelial cells.34 Based on our CAPE effect studies on VEGF levels after brain injury, we assume that CAPE can reduce VEGF levels compared to the group of rats without CAPE treatment by reducing vascular permeability after brain injury.
ConclusionFrom this study, we conclude that the rats with brain injury that were given CAPE had lower VEGF levels than the ones without CAPE treatment. VEGF is an indicator of vascular permeability activity in the blood, and CAPE can reduce vascular permeability.
DeclarationThe research for this study was done in partial fulfillment of the requirements for RAN's PhD at the Hasanuddin University.
ConsentThis manuscript does not involve human participants, human data, or human tissue.
FundingNo funding or sponsorship.
Author contributionsRAN and HW designed the study, collected the data, analyzed and interpreted the results and wrote the manuscript. AAI, MH, MNM, and BB (supervisor), WS, CK, Pri, KIN (co-supervisor) contributed to the study design, data analysis and the subsequent drafts of the manuscript. RAN, AAI, WS, Pri and MF performed head trauma treatment and surgery. All authors read and approved the final manuscript.
Conflict of interestThe authors declare no conflict of interest.
A higher appreciation to all staff from the Laboratory of Molecular Biology and Immunology, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia.
Peer-review under responsibility of the scientific committee of the Technology Enhanced Medical Education International Conference (THEME 2019). Full-text and the content of it is under responsibility of authors of the article.