Technology Enhanced Medical Education International Conference (THEME 2019)
Más datosStromal Vascular Fraction cells (SVFs) are adipose tissue stem cells consisting of several types of cells, including Adipose Derivate Stem Cells (ADSCs). Platelet Rich Plasma (PRP) is a stem cell originating from blood that stimulates wound healing. This study aims to compare the acceleration of the healing process between a combination of PRP and SVFs, PRP, SVFs, and Vaseline using an animal model.
MethodsThis experimental study employed experimental method with post-treatment tests using a Wistar strain rat. The rats were divided into four groups: (1) PRP; (2) SVFs; (3) PRP and SVFs; and (4) Vaseline. Each group was given one treatment, and tissue samples were taken on days 0, 4, 7, 10, and 14.
ResultsIn the combined PRP and SVFs group, epithelialization, Polymorphonuclear (PMN) counts, fibroblast counts, and angiogenesis were found to be significantly increased. Compared with the other groups, the combined PRP and SVFs group was found in complete epithelialization on day 10 and slightly better at collagen synthesis compared to another groups. In the combined PRP and SVFs group, angiogenesis was found to be better than the other groups on day 4. PMN counts peaked on day 7 and began to decrease on day 10 in the PRP, SVFs, and the combined PRP and SVFs groups. Decrease of granulation mostly occurred on day 4 in the combined PRP and SVFs groups.
ConclusionsThere was a significant increase in epithelialization, tissue granulation, and collagen synthesis in the combined PRP and SVFs group compared to another group.
Burn trauma is defined as damage to the skin and underlying tissues caused by heat, chemicals, or electricity. Every year in the United States 450,000 people receive medical treatment for burn trauma. An estimated 4000 people die each year from fires and burn trauma. The goal of wound management is to provide fast and comprehensive treatment, mainly for functionality and esthetics.1
Platelet Rich Plasma (PRP) originates from blood and functions to stimulate endothelial, epithelial, and epidermal regeneration, angiogenesis, collagen synthesis, and tissue healing.2 PRP contains seven types of growth factors, namely, TGF-β, bFGF, PDGFa, PDGFb, EGF, VEGF, and CTGF.3
Stromal Vascular Fraction cells (SVFs) are lipoaspirate components obtained by liposuction of fat tissue. These lipoaspirates contain a large number and variety of stem cells, including Adipose Derived Stem Cells (ADSC), mesenchymal and endothelial progenitor cells, leukocyte subtypes, lymphatic cells, pericytes, T cells, B cells, and vascular smooth-muscle cells. SVFs enhance burn healing through a process that stimulates cell proliferation and vascularization, strengthening the inflammation reaction and increasing fibroblast activity.4–6
In Indonesia, the comparative efficacy between PRP intradermal injections, SVFs, combined PRP and SVFs, and standard wound care (Vaseline) in terms of healing effects in deep dermal burns remains undiscovered. The aim of our study is to prove that the combination of PRP and SVFs accelerates burns healing and is better than other wound care with PRP, SVFs, or with moisture (Vaseline) alone. In this study, we used the parameters of Polymorphonuclear (PMN) counts, fibroblast counts, and thickness of granulation, as well as the formation of epithelialization, collagen, and capillary density as predictors of deep dermal burn healing.
Material and methodsThis study was conducted at the Animal Laboratory and Anatomy Pathology Laboratory faculty of medicine at Hasanuddin University. Adult male Wistar rats (Rattus norvegicus) were enrolled, 45 subjects of 2–3 months old with weights of 150–250 grams. All animal procedures received approval from our local Ethics Commission, number: 251/UN4.6.4.5.31/PP36/2018. The work was also carried out in line with the ARRIVE Guidelines for Reporting Animal Research.7
Skin tissue samples were taken from the rats after termination, on days 0, 4, 7, 10, and 14. Furthermore, skin tissue processing was carried out through paraffin and the hematoxylin–eosin (HE) staining methods. Histopathological examination was carried out on days 0, 4, 7, 10, and 14. The data were processed using SPSS version 23 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.). The effects of combination of PRP and SVFs, PRP, SVFs, and Vaseline administration, in this case, the tissue regeneration of each group was tested by One Way Anova and a post hoc test. p-Values less than 0.05 were considered statistically significant.
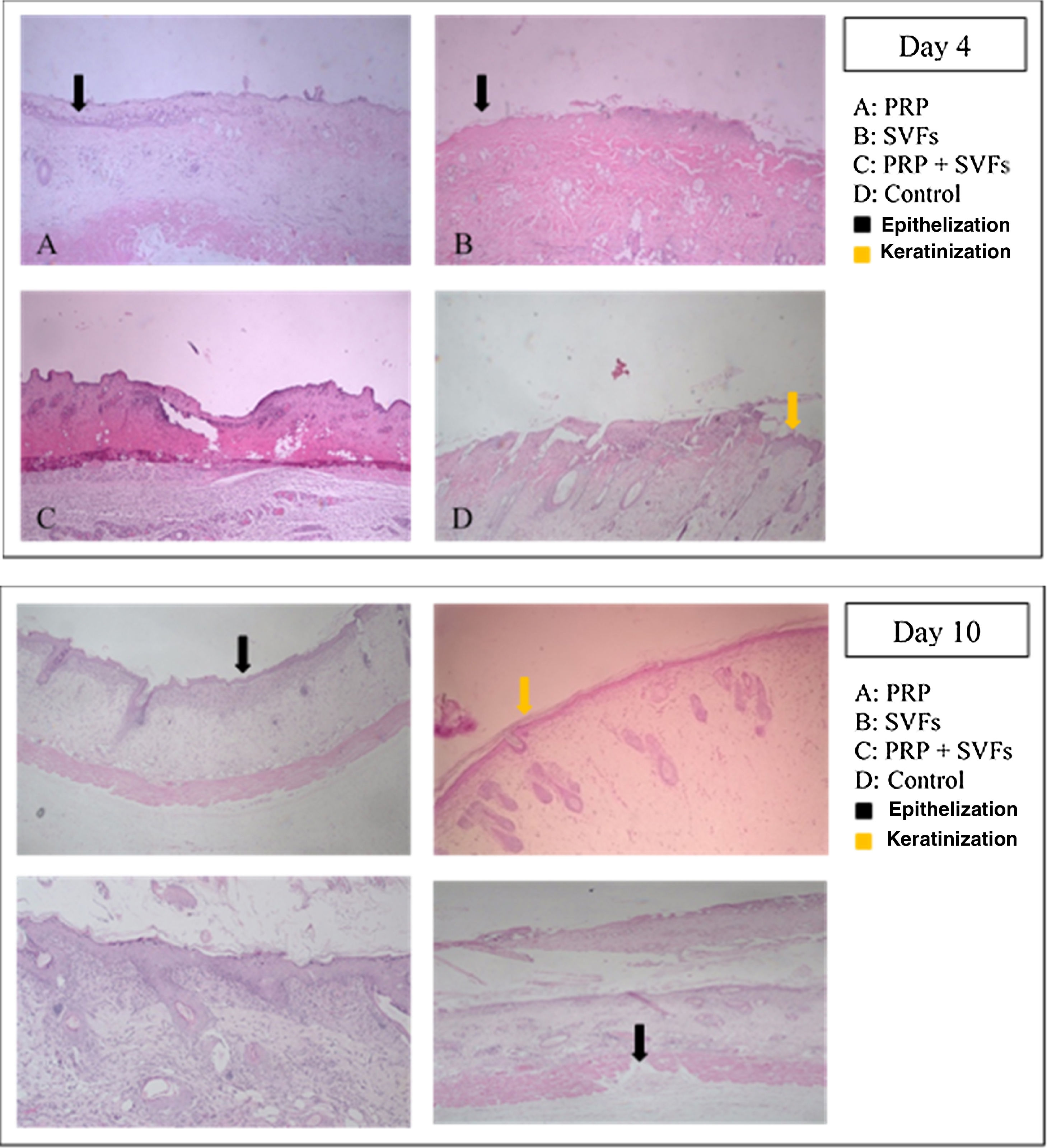
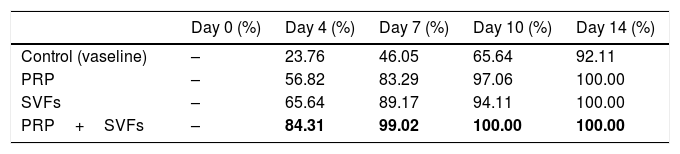
ResultsIn this study, we found that on day 4, the sample of deep dermal burn injury that was given combined PRP and SVFs showed epithelialization closure of 84.31% compared to the other groups: PRP=56.82%, SVFs=65.64%, and standard moisture (Vaseline)=23.76 percent. Complete wound closure (100%) occurred from day 10 in the combined PRP (Platelet Rich Plasma) and SVFs (Stromal Vascular Fractions) groups (Table 1).
We found that epithelialization occurred in the deep dermal burn model on the 10th day, almost completely closed, in the subjects given PRP (97.06%), SVFs (94.11%), and standard moisture treatment (65.64%), with statistical values of p=0.00 and p=0.000 (Fig. 1). Complete closure (100%) occurred in the sample given combined PRP and SVFs compared to the standard moisture treatment (65.64%), with p value=0.000. On the 14th day, groups given PRP, SVFs, and combined PRP and SVFs formed epithelialization and closed completely (100%) compared to the sample treated with standard moisture (92.11%). Statistically, on the 14th day there was no significant difference, with a value of p=0.073 (Table 1).
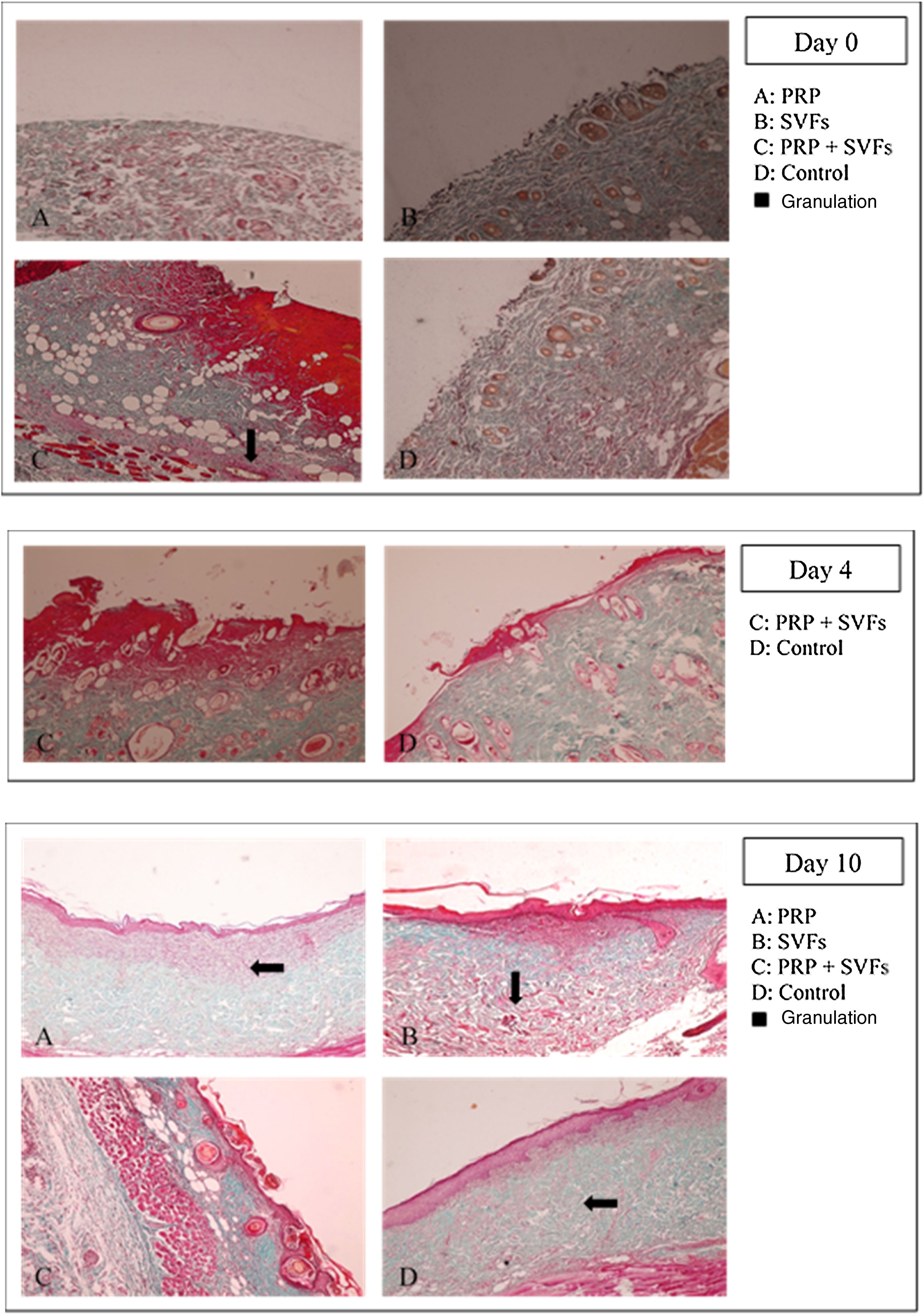
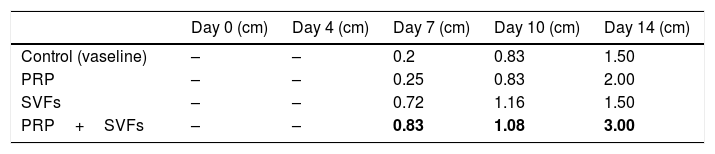
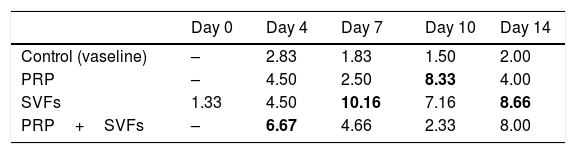
The combined PRP and SVFs group showed increases in collagen thickness occurring at the 7th, 10th, and 14th days of 0.83cm, 1.08cm, and 3.0cm respectively (Fig. 2), whereas those treated with standard moisture were 0cm, 0.83cm, and 1.5cm on the 7th, 10th, and 14th days, respectively. On the 7th day, deep dermal burn trauma models treated with PRP had an average thickness of 0.25cm, and those given SVFs had an average thickness of 0.72cm. On the 14th day, models treated with PRP increased by an average of 2cm, while those given SVFs increased by an average of 1.5cm, and the model treated with standard moisture increased by an average of 1.5cm. This is found statistically significant on the 14th day (p=0.00) (Table 2).
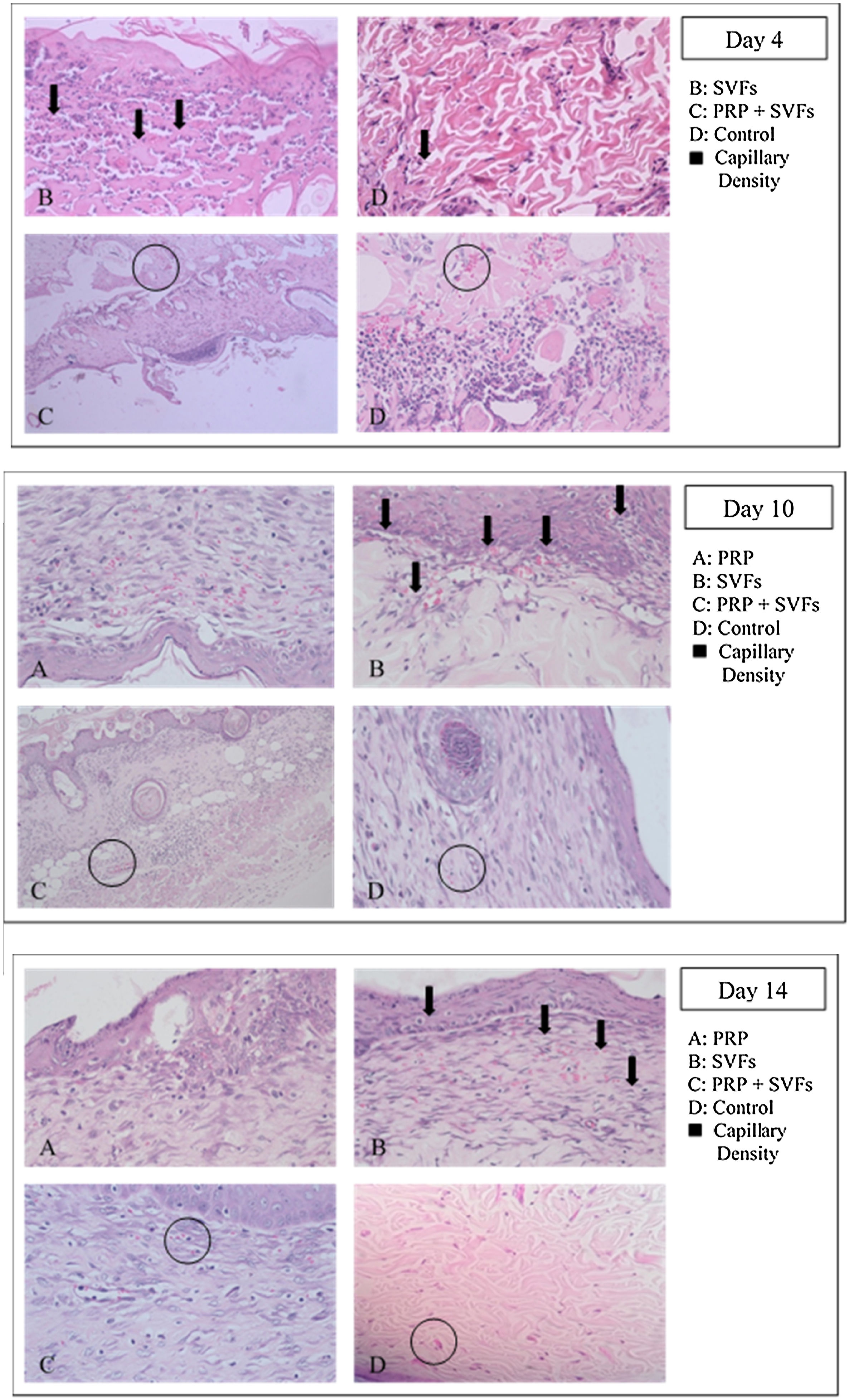
Capillary density of each group compared with the control group increased on the 4th day, then decreased slightly on the 7th day, and settled on the following day; whereas the PRP group fluctuated, first rising on day 4, decreasing on day 7, rising on day 10, and returning on day 14 (Fig. 3). The SVFs group continued to increase until the 7th day, decreased on the 10th day, and slightly increased on the 14th day, whereas the PRP+SVFs group showed a different pattern, increasing rapidly on the 4th day, decreasing continuously until the 10th day, and then increasing on the 14th day. The greatest capillary density was found in the PRP+SVFs group and the SVFs group at the 14th day (Table 3).
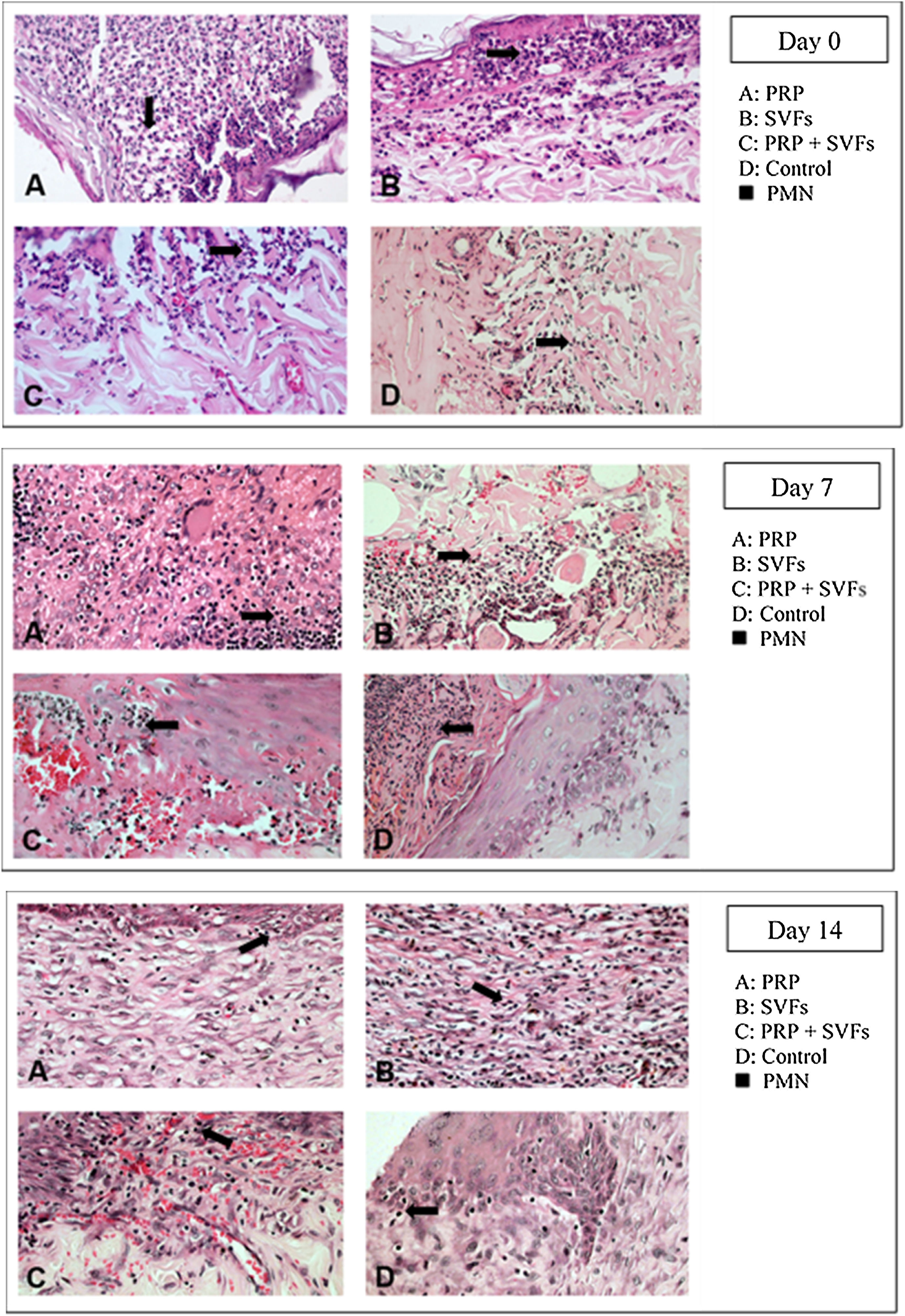
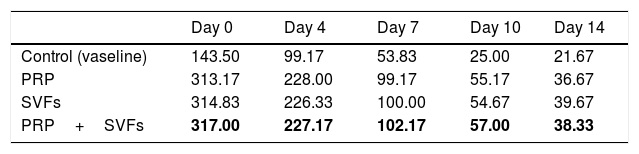
The deep dermal burn trauma models with PRP, SVFs, and a combination of PRP+SVFs show a better amount of PMN compared to the standard moisture care (Vaseline) group on days 0 up until day 14 (Fig. 4). On the 0th day, the numbers of PMN given by the PRP, SVFs, and PRP+SVFs combination group compared with standard moisture treatment were 313.17, 314.83, and 317, respectively, vs. 143.5 with standard moisture. Statistically, the 0th day showed a significant value of p=0.003.
On the 7th day, the numbers of PMNs given by the PRP, SVFs, and PRP+SVFs combination groups were 99.17, 100, 102.17, respectively, while treatment with standard moisture (Vaseline) was 53.83. Statistically, the 7th day showed a significant value of p=0.004. On the 14th day, the number of PMNs in the PRP, SVFs, and PRP+SVFs combination groups was 36.67, 39.67, and 38.33, respectively, compared with standard moisture (Vaseline), which was 21.67. Statistically, the 14th day showed a significant value of p=0.003 (Table 4).
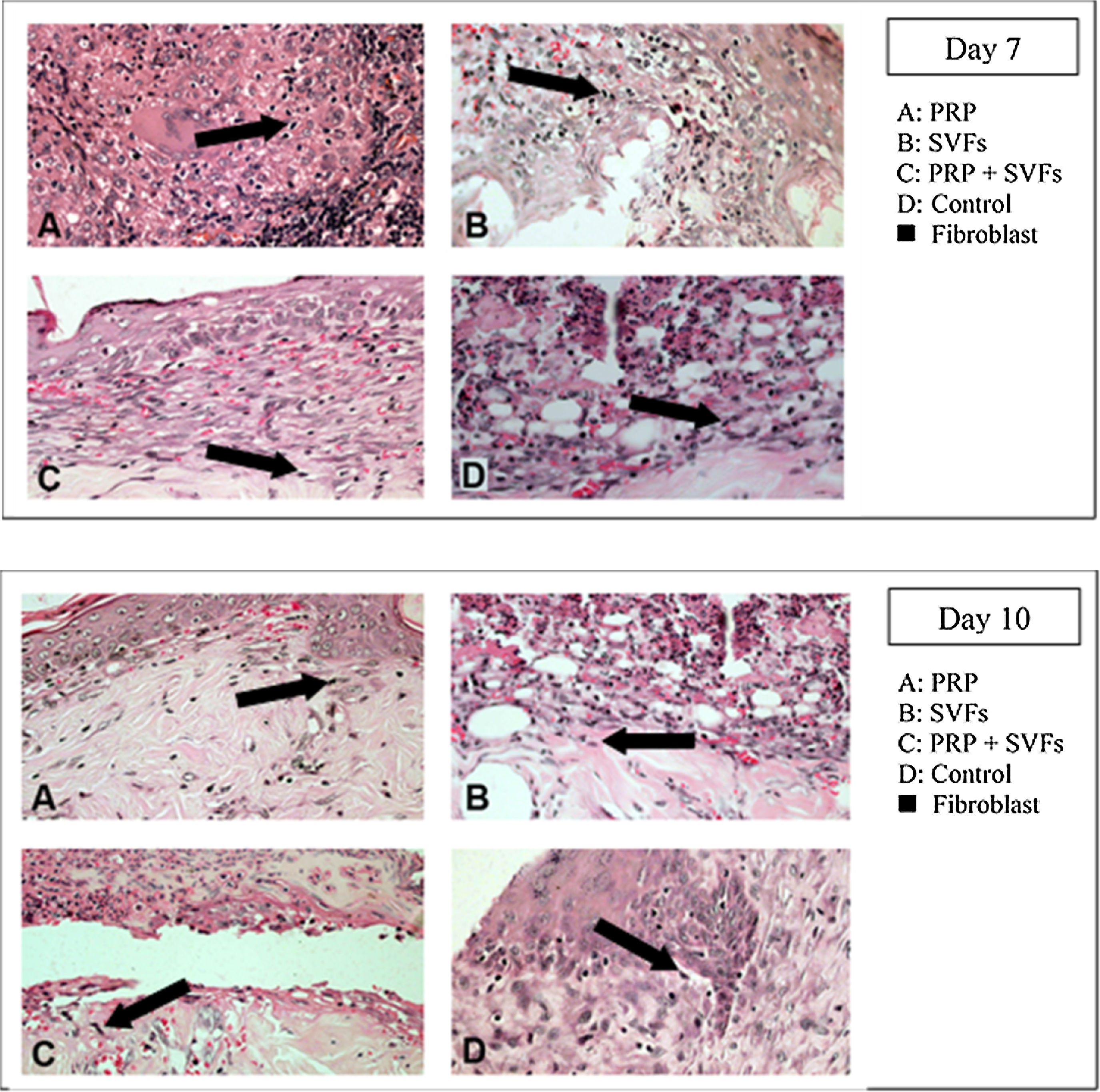
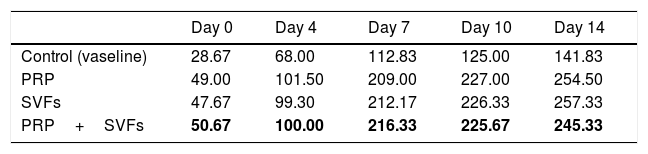
Fibroblast counts on day 7 in the groups given a PRP, SVFs, and PRP+SVFs combination were 209, 212.17, and 216.33, respectively, whereas the count in the standard moisture (Vaseline) treatment group was 112.83. Statistically, the 7th day showed a significant value of p=0.001. On the 10th day, the fibroblasts counts in the groups given the PRP, SVFs, and PRP+SVFs combination were 227, 226.33, and 225.67, respectively (Fig. 5), whereas the count for those given only standard moisture (Vaseline) was 125. Statistically, the 10th day showed a significant value of p=0.004 (Table 5).
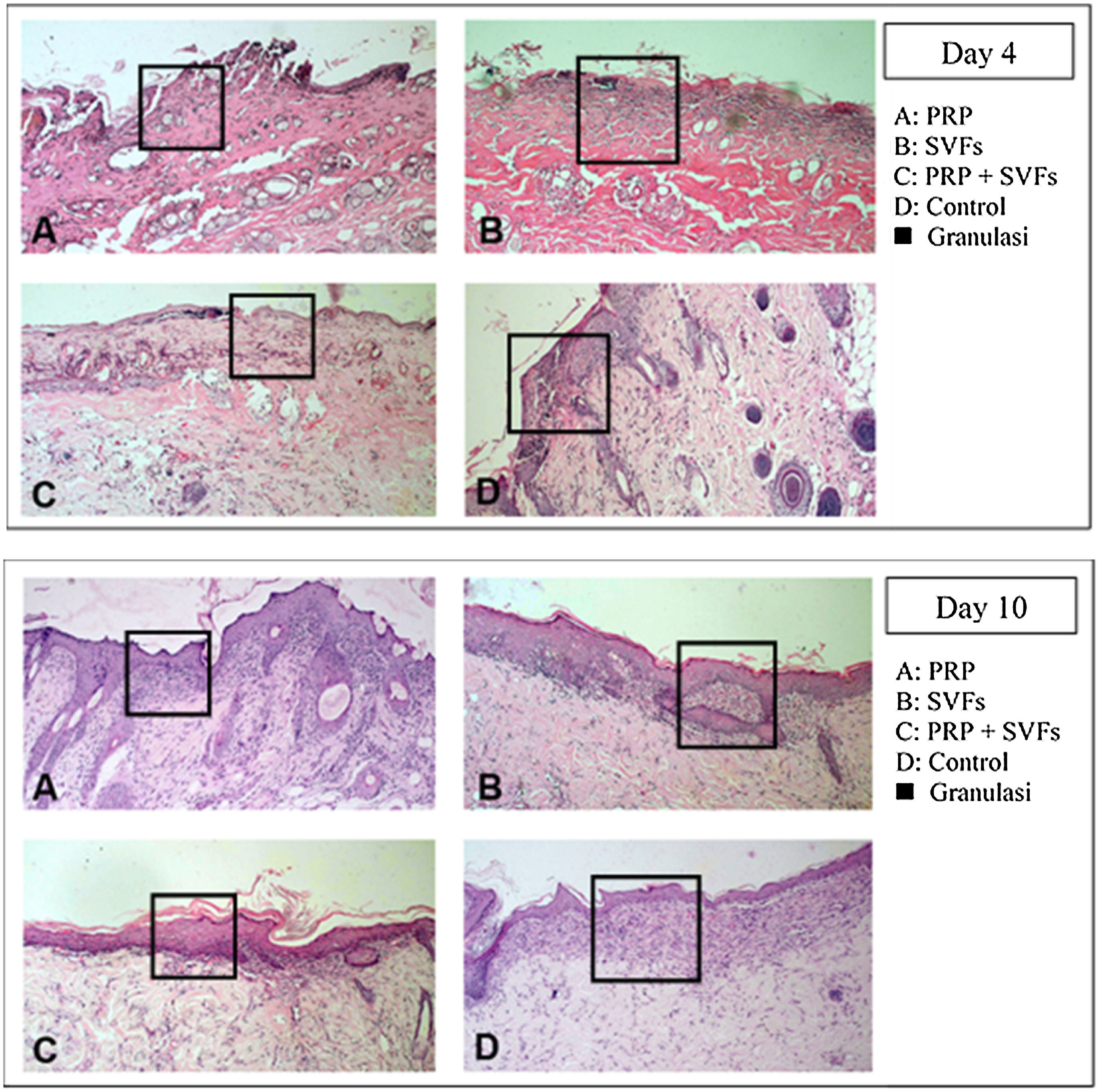
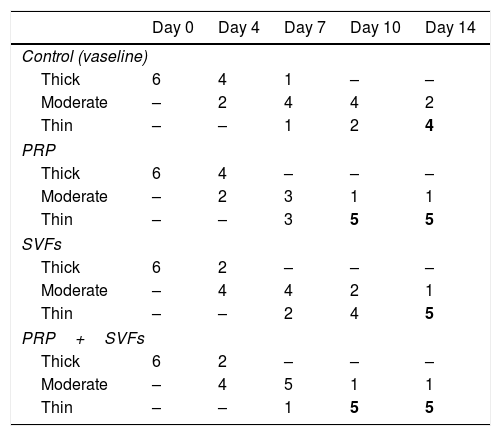
Decreased granulation in all groups occurred on the 4th day, mostly in the SVFs and PRP+SVFs groups. On the 7th day, decreased granulation occurred mostly in the PRP group. On the 10th day, a decrease in granulation occurred mostly in the PRP and PRP+SVFs groups (Fig. 6). On the 14th day, a decrease in granulation occurred similarly in the three groups other than the control group (Vaseline) (Table 6).
Percentage decrease in granulation of each group.
| Day 0 | Day 4 | Day 7 | Day 10 | Day 14 | |
|---|---|---|---|---|---|
| Control (vaseline) | |||||
| Thick | 6 | 4 | 1 | – | – |
| Moderate | – | 2 | 4 | 4 | 2 |
| Thin | – | – | 1 | 2 | 4 |
| PRP | |||||
| Thick | 6 | 4 | – | – | – |
| Moderate | – | 2 | 3 | 1 | 1 |
| Thin | – | – | 3 | 5 | 5 |
| SVFs | |||||
| Thick | 6 | 2 | – | – | – |
| Moderate | – | 4 | 4 | 2 | 1 |
| Thin | – | – | 2 | 4 | 5 |
| PRP+SVFs | |||||
| Thick | 6 | 2 | – | – | – |
| Moderate | – | 4 | 5 | 1 | 1 |
| Thin | – | – | 1 | 5 | 5 |
The results showed that burn trauma treated with a combination of PRP and SVFs provided better healing of deep dermal burn trauma than when treated with PRP and SVFS, and they worked much faster than standard moisture treatments.
Re-epithelialization is the reshaping of the epithelial layer from the wound surface until the wound is closed, which is characterized by the occurrence of epithelial cell migration on the surface of the tissue, at the edge of the wound, or in the surrounding healthy skin.8,9 Migration can occur several hours after the injury. Epithelial cells need viable tissue for the migration process. Epithelial cells at the edge of the wound proliferate on the 2nd or 3rd day after the injury to provide enough cells for the migration process.8–11
This study of deep dermal burns showed that epithelialization occurring on the 4th day after the burns in the PRP+SVFs group (84.31%) was faster than in the PRP (56.82%), SVFs (65.64%), and standard moisture groups (23.76%). Epithelialization was almost complete on the 10th day in the PRP+SVFs combination group (100%) compared with PRP (97.06%), SVFs (94.11%), and standard moisture (65.64%). Our study is comparable with Atalay et al. (2014) who reported significantly higher values of epithelization in burn trauma with SVF treatment compared with controls. Increased epithelial thickness was also reported in skin defects injected intradermally with ADSC culture compared with wounds injected with PBS (phosphate-buffered saline). The study shows that increased epithelialization can also be obtained when ADSC is used without prior expansion, but in an SVF uncultivated form, on days 13, 16, and 21.12–14
A study conducted by Irma in 2012 reported that the administration of topical Platelets Rich Plasma (PRP) increased the process of tissue regeneration in white mice. This is because PRP is an autologue of platelets in plasma in smaller portions. PRP contains seven active protein growth factors that are released during the wound healing process. Growth factor levels remain stable after synthesis of PRP. Platelet concentrations in PRP can increase eight times from platelet levels in the blood, and that causes the growth factor levels in PRP to also increase eight times.2,3,15–17
Study by Roos et al. (2015) reported that the application of PRP to dermis with burn injury showed significantly better reepithelization rate than controls. Also, younger patients showed better epithelialization in the area applied with PRP.15 Similar results were found in the study of Kazakos et al., showing the effectiveness of PRP in the management of acute wounds and burns.18 Theoretically, PRP can be useful for burns. However, PRP induces a severe inflammatory response to burn injury and may stimulate the formation of excessive granulation tissue or hypertrophic scarring.18,19 An excess of granulation tissue is avoided in burn injuries with superficial or partial defects, but the granulation tissue may be needed in burns with deep defects (full thickness burn).20 However, this effect was not found in the study conducted by Singh et al. on second-degree burns, which demonstrated that PRP applications show faster epithelialization achievement, wound healing without hypertrophic scars, and ability to reduce the separation of skin grafting.21
A study by Cervelli et al. (2010) reported a combination of PRP, autologous ADSCs, and hyaluronic acid as dressings for tissue regeneration and epithelization of wounds in the lower limbs significantly reduced healing time in 30 patients. Furthermore, the authors report fewer drugs used and improvement in the quality of life of these patients.22
Collagen plays a major role in wound healing and is an important component of connective tissue that provides a structural framework for tissue regeneration. After injury, protein synthesis occurs immediately in the wound area.23,24 Collagen is the main extracellular protein found in granulation tissue in wounds. Collagen synthesis is propagated by growth factors and cytokines, namely, PDGF, FGF, TGFβ, and IL-1, IL-4, and IgGI, produced by leukocytes and lymphocytes at the time that collagen synthesis occurs. In addition, the most important components in collagen synthesis are fibroblast cells.25,26
The results of this study show that beginning on the 7th day, collagen began to form in burn wounds treated with PRP and SVFs compared to those treated with standard moisture (Vaseline). Statistically, the comparison was significant on day 7 in the PRP group and the SVFs group. On the 7th day, there was an increase compared to previously (3rd day). This was in accordance with the theory that macrophages increase collagen synthesis (appear 48–96hours after injury), which stimulates the growth factor and attracts fibroblasts to the wound. On the 14th day, there was an increase in the thickness of collagen compared to the 10th day. This might be due to starts in the remodeling phase where extracellular matrix (ECM) degradation begins including collagen by the matrix metalloproteinase (MMP) enzyme produced by myofibroblast as part of wound healing.27,28 If collagen is still greatly increased in the remodeling phase, the likelihood of hypertrophic scars is greater.24,28,29
Rigotti et al. (2016) reported that therapy with expanded adipose-derived stem cells and fat rich in SVFs easily modified the pattern of collagen and elastic tissue in the dermis. The combination of SVFs and PRP also induces modifications of papillary dermis, reticular dermis, and the dermo-epidermal junction. In papillary dermis before treatment, oxytalan and elastic fibers are rarely seen. After treatment there is an increase in oxytalan and elastic fibers that can be seen in orcein staining.30
Gokulakrishnan et al. (2016) found that on the 7th day in the dermal area there was a small amount of immature collagen fibers with incomplete thickness. Moderate neovascularization with immature collagen was seen in experimental animal studies comparing ACS to PRP. On the 14th day, the surface of the scar decreases due to the rapid contraction of the wound and hyperplasia of the epidermis; in the dermis, there is an increase in mature collagen and immature collagen at the center of the wound. PRP and SVF stimulate collagen synthesis and maturation, thereby increasing elasticity of the healing skin.31
An important part of successful wound healing is how fast microcirculation functions again in the hypoxic tissue, which occurs through the process of angiogenesis, which is the lengthening of new blood vessels from previously existing blood vessels. Under normal conditions, tissue cannot grow to exceed 1–2mm in diameter without neovascularization, this determined by the limits of the diffusion of oxygen and metabolites such as glucose and amino acids.32,33
This study showed that capillary density in each group and the control group only increased on the 4th day and then decreased; whereas in the PRP group it fluctuated, first rising on day 4, then decreasing on day 7, rising on day 10, and returning again on day 14. The SVFs group continued to increase until the 7th day, decreased on the 10th day, and slightly increased on the 14th day, whereas the PRP+SVFs group demonstrated a different pattern, increasing rapidly on the 4th day, decreasing continuously until the 10th day, and then increasing on the 14th day. The average capillary density of PRP, SVFs, and PRP+SVFs groups was higher than in those treated with standard moisture on the 4th, 7th, 10th, and 14th day of evaluation, even though it was only statistically significant on the 10th day with p=0.027 and on the 14th day with p=0.02. This may be due to oxygenase distribution and metabolic processes that meet the demands throughout the wound area.
At the end of the evaluation day (day 14) there was an increase in the average amount of angiogenesis in the group given PRP+SVFs and a very slight increase in the group treated with standard moisture. This might be due to the process of maturation of the blood vessels still ongoing. One of the PRP+SVFs growth factors, VEGF, is thought to play an important role as a regulator of vasculogenesis and angiogenesis during development, and it also affects the regulation of angiogenesis during the wound healing process.34,35 VEGF is the best and most specific growth factor as a regulator in the physiology and pathology of the angiogenic remodeling process. The expression of VEGF shows a significant increase after injury, with keratinocytes and macrophages being the main producers.36 VEGF plays an important role in the healing process; this is supported by several studies showing that a decrease in VEGF or an increase in degradation will cause defects in wound healing. VEGF stimulates the development of new veins, including smooth muscle cells found in vein walls. Veins form and connect to each other for a complete healing process and to supply the oxygen and nutrients required to form new tissue.36–38
According to the nature of mesenchymal stem cells, SVFs contain heterogeneous cell compositions that have multipotent properties and can differentiate into different tissue types, so they have a strong role in this angiogenesis process.5
In addition, the use of autologous growth factors derived from transfusion of PRP can provide effective support in tissue regeneration because of their ability to stimulate the cell proliferation, differentiation, and neoangiogenesis required for wound healing.39 Administration of a combination of PRP+SVFs showed progressively increasing neovascularization. During PRP therapy, all wounds react with redness due to increased blood supply. The degree of neovascularization was higher on the 7th day of PRP administration.31
This study found that deep dermal burn injury treated with PRP, SVFs, and a combination of PRP+SVFs showed a better number of PMNs compared to the standard moisture treatment (Vaseline) on day 0 to day fourteen. PMN counts (inflammatory phase) reaches its peak on day 7 and starts decreasing on day 10. Our study is in line with a study conducted by Chen et al. (2008) which showed that wounds treated with SVFs have significantly increased numbers of macrophages but no change in the number of granulocytes or CD3 and T cells.40
During the inflammatory phase, the migration of leukocytes by pro-inflammatory cytokines and chemokines and their antagonists takes place. This initial recruitment of inflammatory cells in wound tissue is very important for healing because of the growth factors and cytokines they produce. Atalay et al. (2014) compared infiltration of inflammatory cells in deep partial thickness burn injuries in mice treated with SVF with controls given normal saline. The results showed that although the severity of inflammation was similar in the two groups after days 3 and 7, the statistical peak of inflammation increased significantly in the control group on day 10, which implies that SVFs can reduce inflammation during the wound healing process.12
Cardoso et al. (2016) showed that mononuclear infiltration in the administration of SVFs activates M1 macrophages, which results in the expression of pro-inflammatory cytokines such as IL-1, IL-6, and IL-23. Th17 cells released by IL-17 will induce the recruitment of PMN cells toward the injured area.13 There were increases in monocyte infiltration, but not PMN, on day 7 in the injured area. These results show that the cytokines released by SVFs cells change the activation of macrophages from classic M1 macrophages to M2 regulator macrophages, which will increase the wound healing profile. Activated regulatory macrophages (M2) are known to increase the production of extracellular matrix components and secrete anti-inflammatory cytokines such as IL-10 to control the extension and intensity of inflammation, then they trigger conditions suitable for the process of proliferation of wound tissue.41 While M1 macrophages are involved in microbial decontamination and inflammation of the wounded skin, M2 and M2-like macrophages induce anti-inflammatory, regulatory, and repair functions that aim to close the wound.42
This study found that in the three treatment groups (PRP, SVFs, and PRP+SVFs), the increase in fibroblasts was higher compared to the control group, and the pattern of the three treatment groups did not look different from one another in the number of fibroblasts. This is comparable to the in vitro study of Atalay et al. (2014) regarding ADSC (Adipose-derived Mesenchymal Stem Cells) and supernatant media that induced type I collagen production with human dermal fibroblasts. In that study, the density of fibroblasts was found to be higher on the third day in the SVFs group compared to controls treated with normal saline.12
Stessuk et al. (2016) found that ADSC provided a proliferative stimulus to fibroblasts and keratinocytes within 24h: the higher the concentration of ADSC, the greater proliferative accumulation was provided. ADSC contains a rich factor substance used as paracrine on fibers and keratinocytes such as EGF, FGF, KGF, IGF 1, VEGF, and PDGF; these cytokines are important for skin ulcer repair.16 Another study showed that proliferation of fibroblasts depends on EGF and bFGF, which are present in ADSC and PRP.40,43 Stessuk et al. (2016) found that low PRP concentrations stimulate proliferation and migration of fibers in vitro.16
Lee et al. (2012) showed that ASC-CM (Adipose-derived stem cells) increased proliferation and transcription of type 1 procollagen α1 fibroblasts. Fibroblasts are the most important mesenchymal cells involved in wound healing.44 Fibroblasts in the dermis around the edge of the wound multiply and migrate to the wound chamber. After migrating into the wound, they begin the synthesis of extracellular matrix components, including collagen types I and III, and participate in the formation of granulation tissue.45 Platelet-derived growth factor (PDGF), TGF-β, nerve growth factor, and connective tissue growth factor (CTGF) in the PRP+SVFS combination group function as chemoactractors of fibroblasts.46 EGF, FGF, PDGF, TGF-β, CTGF, and IGF-1 promote fibroblast proliferation, and collagen production from fibroblasts is also stimulated by a number of growth factors, including FGF-2, PDGF, TGF-β, CTGF, and IGF-1.44
Morimoto et al. (2019) reported that fibroblast growth factor 2 (FGF-2 or basic FGF, bFGF) induced granulation tissue formation, epithelialization, and tissue remodeling in wound healing in burn patients and decubitus ulcers.47,48 This study found that deep dermal burn injury treated by PRP, SVFs, and the combination of PRP+SVFs showed better granulation compared to standard moisture treatment only (Vaseline). This is comparable to the study of Hu et al., which concluded that PRP is a potential donor cell in initiating the process of angiogenesis, recruiting blood vessel endothelium in the area and initiating bone regeneration. This is because PRP can increase the mRNA expression of VEGF and PDGF in rat bone marrow to differentiate stromal cells.45 PRP stimulates undifferentiated stem cell proliferation and cell differentiation for tissue regeneration.49
Our study is also comparable to the study of Duran et al. (2006) which reported that PRP significantly increases granulation tissue formation and angiogenesis after the fourth day of treatment with PPP (Platelet Poor Plasma) with PRP-stimulated angiogenesis, reepithelization, tissue granulation, and collagen synthesis compared to topical SP (Serum Physiologic). They observed a significant increase in the formation of angiogenesis and granulation of tissues in the PRP group compared to the PPP group.50 A study conducted by Van Pham et al. (2013) showed that PRP with ADSC significantly increased cartilage formation in murine mouse models compared with those not given ADSC.35
ConclusionThis study found that PRP, SVFs, and PRP+SVFs stimulate angiogenesis, re-epithelialization, tissue granulation, collagen synthesis, and PMN counts better than the Vaseline group, and there was a significant increase in epithelialization, tissue granulation, and collagen synthesis in the PRP+SVFs combination group compared to PRP or SVFs only.
Conflict of interestThe authors declare no conflict of interest.