Technology Enhanced Medical Education International Conference (THEME 2019)
Más datosThe study aimed to assess the mean velocity of blood flow in the posterior cerebral artery and basilar artery in Parkinson's disease with sleep disorders in the neurology outpatient clinic of RSUP. Dr.Wahidin Sudirohusodo Makassar.
MethodsThis study was conducted on Parkinson's patients with sleep disorders detected by using the Pittsburgh Sleep Quality Index (PSQI) score, Insomnia Severity Index (ISI), Epworth Sleepiness Scale (ESS). Subjects who experienced two disorders of the three scores performed a transcranial doppler examination to calculate the mean velocity of blood flow in the posterior cerebral artery and basilar artery.
ResultsSeven subjects met the inclusion criteria with an average age (60.1±6.89 years), four male patients, and three female patients. The most significant score was PSQI showing mild to severe poor sleep quality (11.1±4.74) and the ESS score showing mild to moderate sleepiness (8.57±4.92). The mean velocity of blood flow decreased in the posterior cerebral artery and basilar artery. Posterior cerebral artery; PSQI scale; right (27.5±3.59), left (27.5±6.41), right ESS scale (28.7±3.30), left (27.6±9.29) basilar artery; PSQI and ESS scale (19.5±4.9).
ConclusionParkinson's disease with sleep disorders in the PSQI and ESS scores show a decrease in the mean velocity of blood flow in the posterior cerebral artery and basilar artery. It is according to the supply of blood flow to substantia nigra pars reticularis and pons that play a role in non-motor symptoms of Parkinson's disease.
Parkinson's disease is a neurodegenerative disease characterized by slow progressive symptoms in the form of resting tremors, rigidity, akinesia/bradykinesia, and postural instability. This disease is a neurodegenerative disease that is most common after Alzheimer's disease.1,2
The nonmotor aspects of Parkinson's disease are common and significantly affect the daily lives of individuals, putting pressure on patient safety as well as an economic burden on society. Better care for these aspects of the disease can reduce suffering. One of the main nonmotor aspects is sleep disorders. Sleep-related problems in PD can be divided into disorders of sleep and disorders of wakefulness. Sleep disorders include nocturnal akinesia, insomnia, sleep fragmentation with increased periods of wakefulness during the night, nocturia, restless leg syndrome (RLS), periodic limb movement syndrome (PLMS), RBD, sleep apnea, and parasomnias. Disturbances of wakefulness include EDS and sleep attacks (SA). Sleep disorders can be assessed, including using Pittsburgh Sleep Quality Index (PSQI), Insomnia Severity Index (ISI), Epworth Sleepiness Scale (ESS).1,3
Most of the nonmotor symptoms in the early stages are related to the complex intrinsic pathogenesis process, which involves not only striatonigral pathways and dopaminergic neurotransmitters but also extranigral pathways and various non-dopaminergic neurons. It is consistent with the Braak hypothesis, which carries the concept of caudo-rostral ascending. According to the neuropathological substrate on the anatomical structure and the Braak stadium, sleep disturbance is caused by abnormalities in the pons, locus coeruleus, raphe nucleus, lateral tegmental nucleus, and also in mesencephalon; especially the basal ganglia namely the substantia nigra. The arteries that supply the basal ganglia in the medial substantia nigra along with the corticospinal tract from crus cerebral receive supply from the paramedian branches of the basilar artery originating from the vertebral artery. In contrast, the lateral portion of the substantia nigra is supplied by the thalamoperforata artery and the posterior choroidal, which are branches from the posterior cerebral artery. The pons is mostly supplied by pontine arteries, which are branches of the basilar arteries originating from the vertebral arteries.4,5
Transcranial doppler ultrasound (TCD) is a noninvasive, nonionizing, cheap, portable and safe technique, a test used to measure the velocity of blood flow in blood vessels of the brain indirectly, by comparing it to the velocity of erythrocytes in the basal cerebral arteries In the Willis Circulus, the probe is placed in particular areas of the scalp (can be in the area of the foramina or thin bone) called the acoustic window. Acoustic window including; transtemporal window to assess the medial cerebral artery, anterior cerebral artery and posterior cerebral artery, transorbital window to assess the ophthalmic artery and internal carotid artery of the siphon section and transforaminal window to assess the basilar artery and vertebral artery.6,7
Because the pathology of Parkinson's disease with sleep disorders is in the basal ganglia, especially the substantia nigra and the pons, here we will see the mean velocity arterial blood flow using transcranial doppler in the arteries that supply the region; namely the posterior cerebral artery (PCA) and the basilar artery (BA).
MethodResearch locationThis research was conducted at the Outpatient Poly Neurology Hospital of Wahidin Sudirohusodo Makassar General Hospital, in May–June 2019. The sample used in this study were all Parkinson's sufferers who underwent transcranial doppler examinations.
Types and sources of dataData to be measured from the sample are data related to demographic data, as well as a score of three rating scales, namely the Pittsburgh Sleep Quality Index (PSQI), Insomnia Severity Index (ISI), Epworth Sleepiness Scale (ESS).
Data collection techniqueThe research sample was determined by consecutive sampling in which all Parkinson's sufferers who came in and had sleep disorders who had previously been tested using a questionnaire; Pittsburgh Sleep Quality Index (PSQI), Insomnia Severity Index (ISI), Epworth Sleepiness Scale (ESS). Seven samples fulfill the inclusion and exclusion criteria.
ResultThis study was to observe at the mean velocity of blood flow examined by transcranial doppler in the PCA and BA in patients with Parkinson's disease with sleep disorders at Wahidin Sudirohusodo General Hospital. This study obtained patients with Parkinson's disease with sleep disorders whose scores were measured using 3 rating scales, namely the Pittsburgh Sleep Quality Index (PSQI), Insomnia Severity Index (ISI), Epworth Sleepiness Scale (ESS). In this study, a total sample of 7 people was obtained that fulfills the inclusion criteria. Characteristics of the sample include gender, age, PSQI score, ISI score, and ESS score.
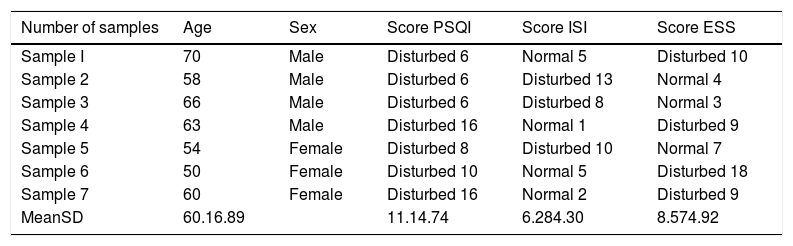
Based on Table 1 obtained consisted of 4 male samples and three female samples. When viewed from the youngest age, sample 6 is 50 years, the oldest is sample 1, which is 70 years, while the mean age is 60.1±6.89 years; the lowest PSQI score was 6, the highest was 16, the mean was 11.1±4.74; the lowest ISI score is 1, the highest is 13, the mean is 6.28±4.30; the lowest ESS score was 3, the highest was 18, the mean 8.57±4.92.
Demographic characteristics of the sample.
| Number of samples | Age | Sex | Score PSQI | Score ISI | Score ESS |
|---|---|---|---|---|---|
| Sample I | 70 | Male | Disturbed 6 | Normal 5 | Disturbed 10 |
| Sample 2 | 58 | Male | Disturbed 6 | Disturbed 13 | Normal 4 |
| Sample 3 | 66 | Male | Disturbed 6 | Disturbed 8 | Normal 3 |
| Sample 4 | 63 | Male | Disturbed 16 | Normal 1 | Disturbed 9 |
| Sample 5 | 54 | Female | Disturbed 8 | Disturbed 10 | Normal 7 |
| Sample 6 | 50 | Female | Disturbed 10 | Normal 5 | Disturbed 18 |
| Sample 7 | 60 | Female | Disturbed 16 | Normal 2 | Disturbed 9 |
| MeanSD | 60.16.89 | 11.14.74 | 6.284.30 | 8.574.92 |
PSQI, Pittsburgh Sleep Quality Index; ISI, Insomnia Severity Index; ESS, Epworth Sleepiness Scale.
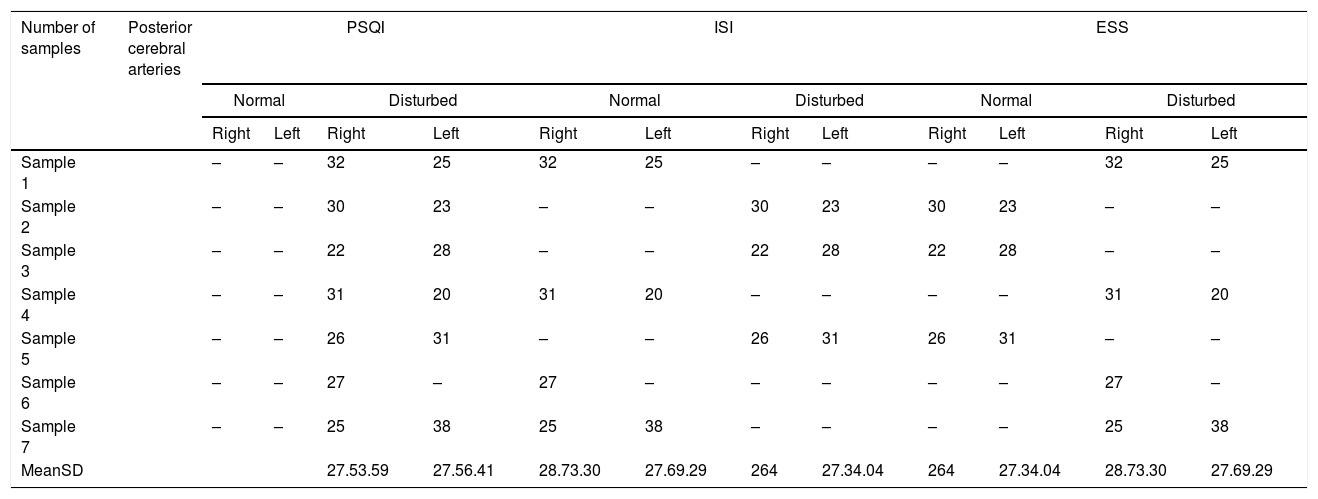
Table 2 shows the mean values of the right and left PCA on each score assessed covering the Pittsburgh Sleep Quality Index (PSQI), Insomnia Severity Index (ISI), Epworth Sleepiness Scale (ESS).
Mean flow velocity of the posterior cerebral arteries.
| Number of samples | Posterior cerebral arteries | PSQI | ISI | ESS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Disturbed | Normal | Disturbed | Normal | Disturbed | ||||||||
| Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | ||
| Sample 1 | – | – | 32 | 25 | 32 | 25 | – | – | – | – | 32 | 25 | |
| Sample 2 | – | – | 30 | 23 | – | – | 30 | 23 | 30 | 23 | – | – | |
| Sample 3 | – | – | 22 | 28 | – | – | 22 | 28 | 22 | 28 | – | – | |
| Sample 4 | – | – | 31 | 20 | 31 | 20 | – | – | – | – | 31 | 20 | |
| Sample 5 | – | – | 26 | 31 | – | – | 26 | 31 | 26 | 31 | – | – | |
| Sample 6 | – | – | 27 | – | 27 | – | – | – | – | – | 27 | – | |
| Sample 7 | – | – | 25 | 38 | 25 | 38 | – | – | – | – | 25 | 38 | |
| MeanSD | 27.53.59 | 27.56.41 | 28.73.30 | 27.69.29 | 264 | 27.34.04 | 264 | 27.34.04 | 28.73.30 | 27.69.29 | |||
Based on the PSQI scale that is disrupted with the highest mean right PCA 32 and the lowest 22, the mean 27.5±3.59; left highest PCA 38, lowest 20, mean 27.5±6.41. On the standard ISI scale, the highest right PCA value was 32. The lowest was 25, the mean 28.7±3.30; left highest PCA 38, lowest 20, mean 27.6±9.29; ISI scale disrupted right highest PCA value 30, lowest 22, mean 26±4; left highest PCA 31, lowest 23, mean 27.3±4.04. On a standard ESS scale the highest right PCA value is 30, the lowest is 22, the mean is 26±4; left highest PCA 31, lowest 23, mean 27.3±4.04; ESS scale impaired right highest PCA value 32, lowest 25, mean 28.7±3.30; left highest PCA 38, lowest 20, mean 27.6±9.29.
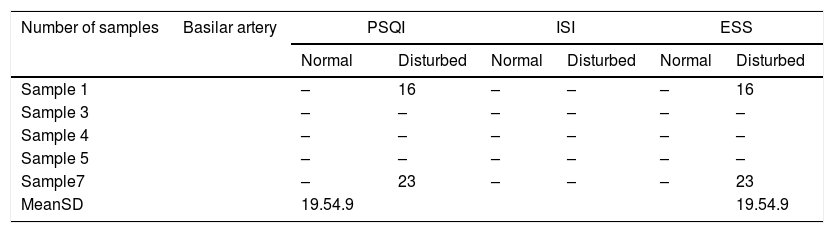
Table 3 shows the mean values of the BA on both the PSQI and ESS scale; the highest 23 and the lowest 16, with a mean of 19.5±4.9.
DiscussionOn the three scoring scales that have been measured, all samples have sleep disorders. Especially the PSQI scale in which all samples (7 samples) have sleep disorders that show mild to severe poor sleep quality (11.1±4.74); on the ISI scale only three patients experienced mild insomnia (6.28±4.30), four patients did not experience sleep disturbance; and ESS 4 patients who showed mild to moderate sleepiness (8.57±4.92), while three patients were normal. It is based on the assessment score that shows that patients with Parkinson's disease experience more sleep quality disorders and daytime drowsiness, compared to insomnia.
Sleep disorders in Parkinson's disease cause degeneration of the dopaminergic and non-dopaminergic systems. Sleep disorders are mostly caused by progressive disease processes that damage the thalamocortical arousal and affect the central regulation of sleep in the brain stem. It is what disrupts REM and non-REM architecture. Saper shows a flip-flop-switch pattern governing the sleep-wake cycle which shows the brain can “off” (fall asleep by activating the ventrolateral preoptic area, sleep promoter) or “on” (in negotiations related to the protection of the tuberomammillary nucleus (TMN), the activation area along with locus coeruleus (LC) and raphe nuclei (DRN)). The second internal rhythm of the “switch” controlled by the arrangement of the anterior hypothalamic suprachiasmatic nucleus (SCN; ‘biological clock’). In Parkinson's disease, dopaminergic dysfunction and neuronal degeneration can disturb the stability of the switches and regulators. As explained earlier, sleep disorders in Parkinson's disease are due to abnormalities in the brainstem in both mesencephalon and pons. The structure of the mesencephalon includes mainly the basal ganglia in the substantia nigra, thalamus, and hypothalamus. The PCA supplies the mesencephalon, the medial branch of the substantia nigra supplied by the paramedian branch of the BA originating from the VA. In contrast, the lateral part of the lateral nigra supplied by the thalamoperforata artery and posterior choroidal, which are branches of the PCA. Sleep disorders also occur in the structure of the pons include: locus coeruleus, raphe nucleus, the tegmental nucleus which is mostly supplied by pontin arteries which are branches of the BA originating from the VA.3–5
In transcranial droppler examination, the assessment of blood flow to the brain is carried out indirectly, by comparing it to the velocity of erythrocytes in the blood vessels of the basal cerebral arteries in the circulous of Willis. With transcranial droppler, we can assess the mean flow velocity in the basal cerebral artery, including the PCA and the BA, so we want to see the mean flow velocity in the arteries that supply the region, namely the PCA and the BA. The normal value of mean flow velocity in PCA based on age>60 years is in the range of 28.1–43.9cm/s. In the results of this study can be seen in Tables 2 and 3 found that in patients with Parkinson's disease with sleep disorders, the mean flow-velocity rate decreases on all three scales, but based on the score, the most influential are those affected by PSQI and ESS with the percentage of patients who are disturbed by sleep quality and drowsiness compared to insomnia. On the Pittsburgh Sleep Quality Index (PSQI) the right PCA mean is 27.5+3.59cm/s and the left is 27.5+6.41cm/s, and the Epworth Sleepiness Scale (ESS) mean right PCA is 28.7+3.30cm/s and left 27.6+9.29cm/s. The mean flow velocity in the BA based on age is 25.3–38.7cm/s. In patients with Parkinson's disease and with sleep disorders, both the PSQI and ESS scales have decreased BA blood flow with value PSQI and ESS mean flow velocity BA 19.5+4.9cm/s. The regulation center of the sleep in the brainstem both in mesencephalon and pons were abnormal in Parkinson's disease. It is following the anatomy and pathology of sleep disorders. The BA mainly supplies mesencephalon. In particular, the substantia nigra supplied by the PCA and the pons. So that the PCA and BA can assess the type of sleep disturbance based on sleep quality using the PSQI scale and daytime drowsiness on an ESS scale, whereas insomnia is not.6,7
ConclusionIn Parkinson's disease with sleep disorders, the PSQI and ESS scores show a decrease in the mean flow velocity in the PCA and the BA. It is in accordance with the supply of blood flow to the substantia nigra pars reticularis and the pons, which plays a role in the non-motor symptoms of Parkinson's disease.
Conflict of interestThe authors declare no conflict of interest.
Peer-review under responsibility of the scientific committee of the Technology Enhanced Medical Education International Conference (THEME 2019). Full-text and the content of it is under responsibility of authors of the article.