Technology Enhanced Medical Education International Conference (THEME 2019)
Más datosThis study aimed to determine the influence of rTMS on the improvement of quality of sleep in an ischemic stroke patient.
MethodsThis research is analytic experimental on 48 post-ischemic stroke patients registered on the outpatient clinic at Wahidin Sudirohusodo Hospital and also a private clinic, with a consecutive sampling method, randomly divided into control and treatment groups. Sleep quality measurement by the Pittsburgh Sleep Quality Index (PSQI) score, before and after treatment. The statistical analysis of correlation among variables was conducted by using the Mann–Whitney test with a p-value <0.05, which was considered significant.
ResultFrom 48 samples (24 control groups and 24 treatment groups), with PSQI score differences on both groups with 1.58 (±3.08) on the control groups and −1.83 (±1.66) on the treatment group. The decrease of the PSQI score shows improvement in the quality of sleep. p-value is 0.000.
ConclusionThere is an improvement in the quality of sleep on post-ischemic stroke patients treated with rTMS.
Strokes, according to WHO, are clinical manifestations of cerebral dysfunction, both focal and global, which are rapid, last more than 24h, or end in death, in the absence of other causes besides brain circulatory disorders.1 The prevalence of stroke is around 2–18 per 1000 population per year and is the second leading cause of death worldwide after heart disease.2 According to Riskesdas (2018), the prevalence of national stroke based on health diagnosis is 10.9%. However, the highest prevalence of stroke by province based on the diagnosis of health workers is in East Kalimantan, which is 14.7%, followed by D.I.Yogyakarta, 14.6%, and the prevalence of stroke in South Sulawesi is around 10.5%. Furthermore, the risk of stroke recurrence in the first year is around 13–14% and 6% in the following years.2 Post-stroke patients usually complain about sleep disturbances or changes in sleep patterns.3
Post-stroke sleep disorders are a common stroke side effect and occur in about 40–80%.3 Research by Karaca in 2017 showed that 39.1% of the 23 study samples had post-stroke sleep disorders (assessed from the PSQI score).4 It is in line with the research conducted by Pasic et al. about the incidence and type of sleep disorders in post-stroke patients, where 78% of the 200 study samples had sleep disorders.5 Wierzbicki et al. analyzed 43 patients with stroke, and 35 patients among them suffered from sleep disorders.5 Post-stroke sleep disorders are associated with physical activity, dementia, the use of psychotropic drugs, and can also be related to the location of the lesion. However, post-stroke sleep disorders can also be caused by depression or a feeling of fatigue that usually occurs in stroke patients.6
Generally, sleep disturbances in stroke are caused by a disturbance in the cortical or subcortical excitability of neurons, both hypoexitability and hyperexcitability. Lesions in the cortical part of the brain are associated with poor quality of sleep, which is a disturbance during sleep at night, which causes a decrease in total sleep duration of <6h (average sleep time at night in the elderly is 6–8h). At the same time, lesions in the subcortical part are associated with sleep disturbances in the form of excessive drowsiness during the day (although this can also be caused by poor quality nighttime sleep). According to research conducted by Suh et al. in 2014 in 338 study samples, 21% of the samples had a total sleep time of fewer than 6h.7
Poor sleep quality or consequences of sleep disorders that are not treated will affect the healing process and the quality of sleep in stroke patients.7 If left untreated, it can cause disorders including impaired cognitive function, mood changes, excessive drowsiness, and fatigue that can affect stroke rehabilitation, length of hospital stay, and affect stroke output and recurrence. Besides, sleep disorders can also reduce the motivation, energy, and concentration of patients to participate in intensive rehabilitation therapy.8
Sleep disturbances in post-stroke patients can also trigger a second stroke and affect the patient's ability to learn new motor skills and patient functional output. In addition, the quality of sleep and disturbed sleep can also affect the immune system, interfere with the healing process, increase sensitivity to pain, and can also cause depression and anxiety.9 Although there are some data linking sleep disorders with increased patient morbidity and mortality, sleep disorders can affect rehabilitation efforts in stroke patients. Poor sleep quality or abnormal sleep duration, whatever the etiology, interferes with patient rehabilitation significantly through various processes. Sleep disorders interfere with the restoration process, which occurs during sleep. Sleep disorders that often arise in patients with stroke are associated with symptoms of depression, daytime drowsiness, fatigue, and executive dysfunction, all of which can interfere with the rehabilitation of patients. Stroke patients with untreated sleep disorders can reduce the motivation, energy, and concentration of patients to participate in intensive rehabilitation therapy.8
Sleep disorders can be managed by administering pharmacological and nonpharmacological therapy. Administering sedatives (e.g., benzodiazepines) is one of the pharmacological therapy. Nonpharmacological therapies include the use of CPAP in post-stroke sleep disorders patients, especially the type of sleep disorder, and the most recent is the use of repetitive transcranial magnetic stimulation (rTMS).
rTMS is a process of repetitive stimulation in a specific area of the brain that aims to increase plasticity in the brain or increase the brain's ability to reorganize in the form of new interconnections in nerve cells and improve the excitability of nerve cells that are impaired. TMS can be used in different methods to provide direct measurements of cortical excitability. TMS is also used to measure intracortical inhibitor and facilitator mechanisms in the motor cortex. Also, TMS can affect brain function if done repeatedly. rTMS consists of the application of a series of TMS of the same intensity in one area of the brain at specific frequencies that vary between 1 and 20 or more stimuli per second and can modulate cortical excitability and stimulate long-term changes in neuroplasticity.10
This function is also used in the management of sleep disorders. Kunze et al. in 2013 conducted a study by applying rTMS to the dorsolateral prefrontal cortex in ischemic stroke patients with insomnia, and this procedure reduced sleep latency and increased total sleep time.1
Besides, TMS can also activate the corticobulbar system and strengthen the muscles of the upper respiratory dilator so that it can improve the inspiration airflow during sleep. It was evidenced in a study conducted by Nordone et al. in 2014 on 14 patients suffering from OSA. Corticobulbar excitability of the submental muscles is reduced during non-REM sleep. TMS can strengthen submental muscles so that it can improve the function of the upper airway without waking the patient up. Moreover, in OSA patients, there is a disturbance in motor cortex excitability during the day. It can be believed that the partial pressure of arterial carbon dioxide plays a role in several ion channels or metabolic pathways that can be able to modify motor cortex excitability through the role of the inhibitory and excitatory cortical circuits.11
Apart from the benefits posed by rTMS, as far as we know, there have been no studies in Makassar that directly assess the effect of rTMS on the sleep quality of ischemic stroke patients. Therefore this study aims to find out more about the effect of giving rTMS on sleep quality in patients with post-ischemic stroke in the Makassar area. It is essential to identify the characteristics of sleep disorders in post-ischemic stroke patients and to monitor the long-term effects of administration of rTMS on sleep quality in post-ischemic stroke patients. Also, by studying the characteristics of sleep in post-stroke patients, it can provide a better understanding of sleep contributions in the development of comorbid conditions in post-stroke patients.9
MethodResearch locationThis research was conducted in the Wahidin Sudirohusodo Hospital and the satellites and also in Brain Stimulation Clinic, Inggit Medical Center Makassar. This research was carried out for two months, April 2019 to May 2019. The samples used in this research are ischemic stroke patients that come to the outpatient clinic of the hospital and clinic above. The samples were forty-eight ischemic stroke patients who were obtained by consecutive random sampling who met the inclusion and exclusion criteria until the number of samples was fulfilled.
Data collection techniquesThe inclusion criteria are a patient's post-ischemic stroke aged 18–65 years, both male and female, stroke sufferers whose CT scan results are supporting an ischemic stroke (no tumor, abscess or space pressure process), and post-ischemic stroke patient who has a sleep disorder (evidenced by a PSQI score >5). Subjects were excluded from the study if there was impaired cortical function, consume drugs that can affect the sleep cycle, have a history of sleep disorders, impaired consciousness, impaired kidney function, or impaired liver function. Patients were suffering from post-stroke depression as well, as evidenced by the Beck Depression Inventory score.
The sample size required for two-sided testing is obtained by the formula (Lemeshow, 1998) as follows:
Based on the results of research by Jinil Kim et al. (2015) then:
- a.
Type I error rate and type I error direction. The type I (α) error used is 0.05 or 5% with a two-sided direction of type I (α) so that the Z value is 1.96.
- b.
Type II error rate and type II error direction. Type II (α) errors are used at 0.1 or 10% with the one-sided direction of type I (β) so that the Z value is 1.28.
- c.
Mean value. The mean value used is the mean value of PSQI in group 1 of 12.0 and group 2 of 17.7. Based on the minimum number of samples obtained for 24 samples per group.
This research was conducted at the Wahidin Sudirohusodo Hospital in Makassar and its satellites and the Makassar Inggit Medical Center Brain Stimulation Clinic, which held from April 2019 to May 2019. The number of samples collected and fulfilling the inclusion criteria were 48 people. Samples that met the inclusion criteria were divided into two groups, namely the control group (24 people) consisting of ischemic stroke patients suffering from sleep disorders given medical therapy, and the treatment group (24 people) consisting of ischemic stroke patients who had sleep disorders given medical therapy and rTMS.
The PSQI score in the treatment group who received medical therapy and PSQI scores in the control group who received medical therapy and rTMS will be recorded before being given therapy and after being given medical therapy and medical therapy and rTMS. These results can be seen in Tables 1 and 2.
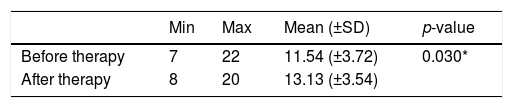
PSQI score before and after given medical therapy on the control group.
| Min | Max | Mean (±SD) | p-value | |
|---|---|---|---|---|
| Before therapy | 7 | 22 | 11.54 (±3.72) | 0.030* |
| After therapy | 8 | 20 | 13.13 (±3.54) |
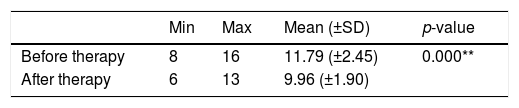
PSQI score before and after given medical therapy and rTMS on treatment group.
| Min | Max | Mean (±SD) | p-value | |
|---|---|---|---|---|
| Before therapy | 8 | 16 | 11.79 (±2.45) | 0.000** |
| After therapy | 6 | 13 | 9.96 (±1.90) |
Table 1 shows that before medical therapy, the minimum range of PSQI score is 7, and the maximum score is 22, with a mean value of 11.54 (±3.72). After medical therapy, the minimum range of PSQI score is 8, and the maximum score is 20 with a mean score of 13.13 (±3.54) in the control group so that the score difference was 1.58 (±3.08). Table 1 also shows that the average PSQI score after therapy is greater than the average score before therapy in the control group. The statistical analysis showed a significant difference (p=0.030) or p<0.05.
Table 2 shows that before medical therapy and rTMS were given, the minimum range of PSQI score is 8, the maximum score is 16, with a mean score is 11.79 (±2.45). After medical therapy, the minimum range of the PSQI score is 6. The maximum score is 13 with a mean score of 9.96 (±1.90) in the treatment group so that the score differences of −1.83 (±1.66) were obtained. Table 2 also shows that the average value of PSQI scores after medical therapy and rTMS is smaller than the average value before therapy in the treatment group, and after testing, the analysis using the paired t-test showed a significant difference (p=0.000) or p<0.05.
The comparison of the PSQI scores in the treatment group and the control group can be seen in Table 3.
Comparison of difference in score changes in control groups and treatment groups.
| Treatment | Control | p-value | |
|---|---|---|---|
| ΔPSQI score | −1.83 (±1.66) | 1.58 (±3.08) | 0.000*** |
Table 3 shows a comparison of the difference in PSQI scores in the treatment group and the control group where the difference in PSQI scores in the treatment group was −1.83 (±1.66) points compared to the control group 1.58 (±3.08) points and after analyzed using the Mann–Whitney test gave a significant difference with the results of p=0.000, meaning that in the treatment group where obtaining medical therapy and rTMS therapy there was a decrease in PSQI scores which showed an improvement in sleep quality compared to the control group who only received medical therapy, showed an increase in PSQI scores which showed a deterioration in the quality of sleep.
DiscussionThis study assessed the effect of rTMS on improving the quality of sleep in post-ischemic stroke patients. Sleep quality was assessed before therapy and after therapy in the control and treatment groups. Sleep quality was determined by PSQI scores measured before therapy in each group and after being given standard stroke therapy in the control group and after being given standard stroke therapy and rTMS in the treatment group.
This study collected 48 samples of ischemic stroke patients who met the inclusion criteria divided into 24 samples as a control group who were given ischemic stroke standard therapy and 24 treatment groups given standard ischemic and rTMS stroke therapy. Differences in PSQI scores before therapy and after therapy in both groups showed significant results. In the control group, there was an increase in PSQI score from 11.54±3.72 to 13.13±3.54, with p 0.030 indicating a deterioration in the quality of sleep in patients after ischemic stroke. As for the treatment group, there was a decrease in the PSQI score from 11.79±2.45 to 9.96±1.90 with p 0.000, which shows an improvement in the quality of sleep in patients after ischemic stroke. The comparison between the differences in PSQI scores in the two groups, if analyzed by the Mann–Whitney test, showed significant results with p 0.000, where this number indicates that there were statistically significant differences in the two groups. It is consistent with a study conducted by Kunze et al. in 2013 which conducted a study by applying rTMS to the dorsolateral prefrontal cortex in ischemic stroke patients with sleep disorders, and this rTMS treatment proved to be able to reduce sleep latency and increase total sleep time. The mechanism plays a role in overcoming sleep disorders by performing rTMS is to improve the excitability of neurons in the cortical and subcortical areas.
The limitation of this study was that there was no longer a follow-up to assess differences in PSQI scores in each group to assess the effectiveness of the therapy given. Another limitation is the questionnaire used in this study, the Pittsburgh Sleep Quality Index, where this score has a sensitivity of 89.6% and specificity of 86.5%, and this questionnaire is subjective in assessing the quality of sleep of patients.
ConclusionThere is an effect of giving standard and repetitive Transcranial Magnetic Stimulation (rTMS) therapy to sleep quality of patients after ischemic stroke. Patients with ischemic stroke who were given standard stroke therapy experienced an increase in PSQI scores, which showed deterioration in sleep quality. Patients with ischemic stroke who were given standard therapy and rTMS experienced a decrease in PSQI scores, which showed improvement in sleep quality.
Conflict of interestThe authors declare no conflict of interest.
The researcher would like to thank all people who have helped in the research process. This gratitude especially goes to our colleagues and patients who have participated in this study—also, the nurses who have been very supportive during data collection.
Peer-review under responsibility of the scientific committee of the Technology Enhanced Medical Education International Conference (THEME 2019). Full-text and the content of it is under responsibility of authors of the article.