Technology Enhanced Medical Education International Conference (THEME 2019)
Más datosSleep is a physiological condition which able to modulate seizures through the involvement of widespread circuits, including thalamocortical pathways. The release of epileptiform can be either activated or inhibited based on the ongoing stage of sleep. Epileptiform release tends to spread during NREM sleep for the synchronization occurs more, while synchronized waves tend to do dominate the REM sleep. The reduction of sleep duration is related to seizure as well as the release of epileptiform waves in epileptic patients.
AimThis study was aimed to know the relationship between insomnia and sleep quality and insomnia with the frequency of seizure in a patient with epilepsy in RSUP Dr. Wahidin Sudirohusodo and its networking hospitals, Hospital of Universitas Hasanuddin as well as Neurologist private clinic in Makassar.
MethodThis was an observational study with a cross-sectional approach involving 31 respondents who met the inclusion criteria. Sleep quality was measured by the PSQI questionnaire (Pittsburgh Sleep Quality Index) during the degree of insomnia with the ISI (Insomnia Severity Index) questionnaire. Samples were taken using a consecutive method.
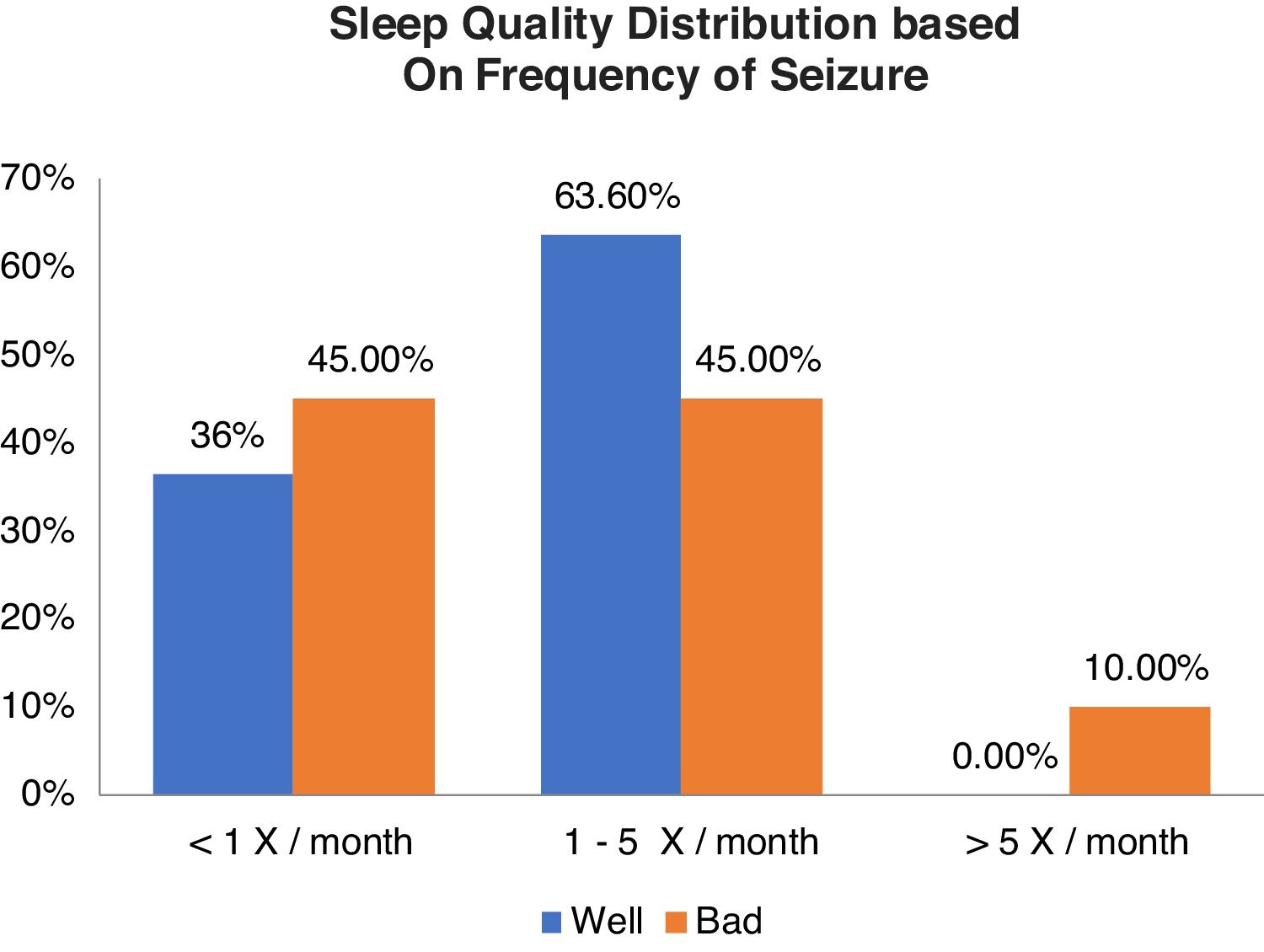
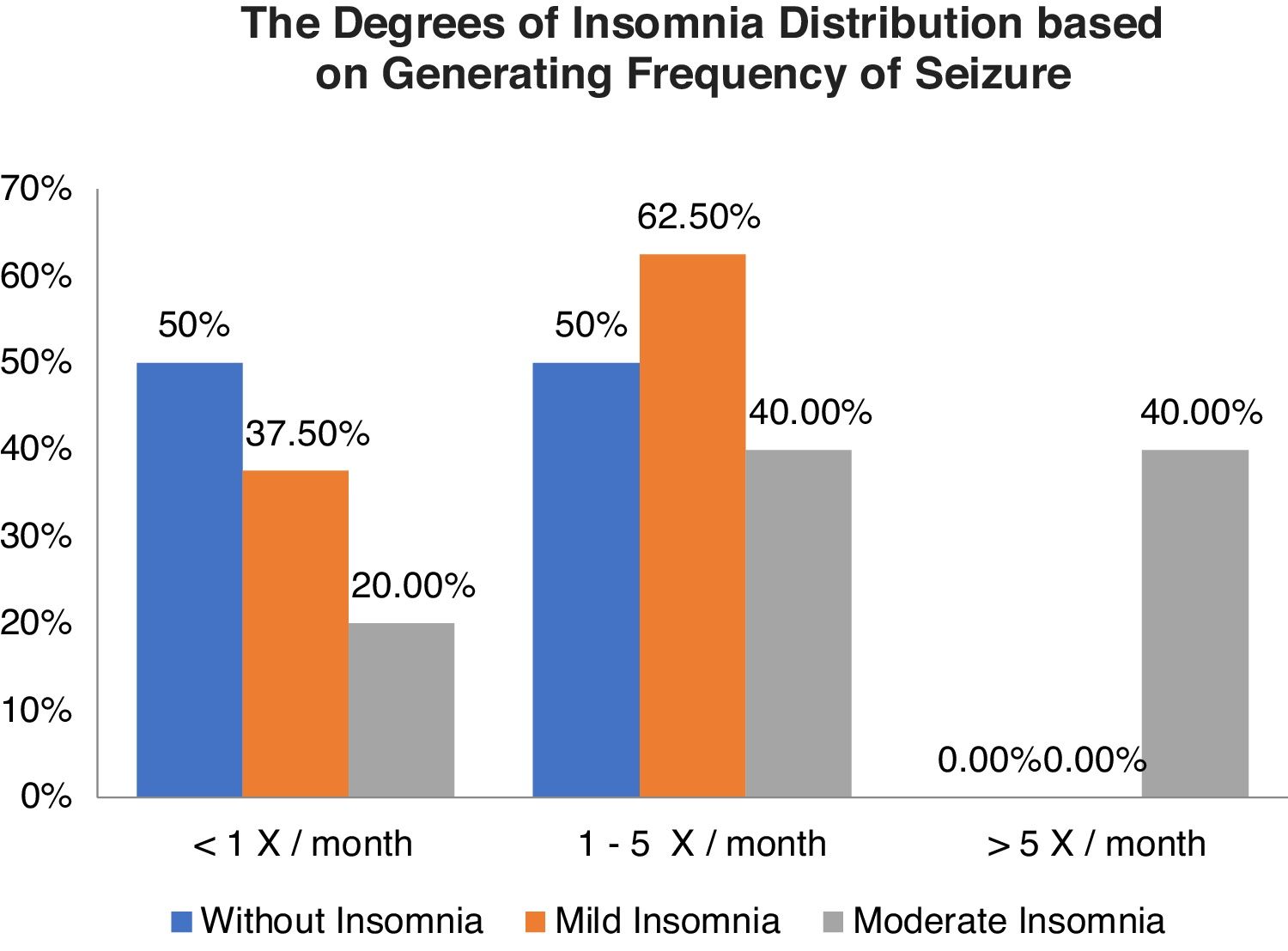
Result11 (33.5%) participants were found to have a good quality of sleep, and 20 (64.5%) others had bad ones. Moreover, 8 (25.8%), 5 (16.1%), and 15 (58.1%) participants were mild, moderate, and without insomnia, respectively. Data analysis using the chi-square test showed p-value 0.427 (>0.05) and 0.020 (<0.05) for the correlation of sleep quality as well as the degrees of insomnia toward the frequency of seizure, respectively.
ConclusionSleep quality is not correlated to the frequency of seizure, while the degree of insomnia was found to be correlated to the frequency of seizure. The frequency of seizure in moderate insomnia was higher compared to the frequency in patients without insomnia.
Epilepsy is one of the oldest neurological diseases, found in all ages and can cause disability and mortality. A minimum of 2 seizures characterizes epilepsy without provocation or two reflex generations with a 24-h more time between seizures. Generating reflexes are seizures that arise as a result of induction by specific trigger factors, such as visual, auditory, somatosensory, and somatomotor stimulation.1
According to WHO, there are an estimated 50 million people worldwide who have epilepsy. The overall prevalence of epilepsy ranges from 2.7 to 41 per 1000 populations, although the majority reported for active epilepsy (i.e., at least one seizure in five years) is in the range of 4–8 per 1000. The prevalence of active epilepsy is generally lower in countries industry than in developing countries, which may reflect a lower prevalence of selected risk factors (mostly infections and trauma), more stringent verification of cases, and the exclusion of provoked and non-provoked isolated seizures.1–3
The incidence of seizures that are not provoked annually ranges from 33 to 198 per 100,000 of the incidence of all epilepsy is 23–190 per 100,000. The overall incidence of epilepsy in Europe and North America ranges between 24 and 53 per 100,000 per year, respectively. In developing countries, the incidence of this disease is higher than in industrialized countries up to 190 per 100,000.2
At the end of the nineteenth century, Gawer commented on the rise in the sleep cycle. In 1929, Langdon-Down and Brain observed that nighttime peak peaks were approximately 2h after sleep and between 4 and 5am, while daytime seizures occur a lot in the first hour after waking up. Berger's discovery of Electrical Enfalo Gram (EEG) in the 1920s was a diagnostic tool to study the relationship between sleep and seizure. Gibbs and Gibbs showed that interictal epileptiform discharge was activated by sleep, and epileptiform activity increased during sleep, so sleeping during EEG recording became the standard activation procedure to date. Janz distinguishes focal and nocturnal epilepsy. Niedermeyer explains the effect of arousal activation in epilepsy.4,5
Sleep is a physiological state that can modulate seizures through the involvement of widely circulated circuits, including thalamocortical pathways. Observations support the effect of sleep on epilepsy that in specific epilepsy syndromes, seizures occur mainly during non-rapid eye movement (NREM). In almost all epileptic syndromes, interictal epileptiform discharges occur more frequently during NREM and less during rapid eye movement (REM). Neuronal synchronization in the thalamo-cortex pathway during NREM causes increased neuronal excitability, leading to more even distribution of focal discharges and facilitating seizures and interictal epileptiform discharges in most people with partial epilepsy. Neuronal synchronization is stopped at wake time (arousal) or transmission to REM sleep, and focal discharges become small.6–8
Sleep quality includes quantitative and qualitative aspects of sleep, such as the length of sleep, the time needed to fall asleep, the frequency of awakening, and subjective aspects such as the depth and severity of sleep. People who sleep frequently are disturbed or shorten their sleep may not get enough from both NREM and REM sleep.9
On the other hand, sleep disorders can trigger seizures. Prevalence is around 24–55% in people with epilepsy. Insomnia is the most common sleep deprivation problem and essential comorbid in epilepsy,10 where its severity interacts poorly with seizure control and has a negative relationship with quality of life.5,11
The main factor that triggers seizures in people with epilepsy is lack of sleep.12 In a recent study, there were more than 97% of epilepsy patients reporting at least one factor that triggered seizures, the top three factors being lack of sleep, both acute and chronic, fatigue, and stress. Sleep deprivation is often used in epilepsy monitoring units to increase the frequency of seizures. An interictal epileptiform release is also evident after lack of sleep. Sleep and sleep deprivation have influences in the onset, frequency, and semiology of seizures, as well as findings in EEG.11,13,14
An epileptiform release can be activated or inhibited depending on the stage of sleep. Epileptiform release tends to spread during NREM sleep because it is more synchronized, whereas, during REM sleep, it appears asynchronous wave release. Reduction of sleep time is associated with seizures and epileptiform wave release in epilepsy patients. The study aims to determine the relationship between sleep quality and the degree of insomnia to the frequency of seizures in epilepsy patients.15
MethodsDesign of researchThe design of this study is an observational analytic study with a cross-sectional approach. The population of the study was all epilepsy patients who were treated at the Neurology Outpatient Dr. General Hospital of Education Wahidin Sudirohusodo and his network, the University of Hasanuddin Teaching Hospital and the practice of a neurologist. The sample of this study was epilepsy patients who treated who met the inclusion and exclusion criteria. Samples are obtained by consecutive sampling method.
Eligibility criteriaThe study was conducted at the Teaching Hospital of Wahidin Sudirohusodo and his network, the University of Hasanuddin Teaching Hospital, and the practice of a neurologist in April–May 2019. The inclusion criteria were adult patients aged 18–55 years old, both male and female, patients who take anti-epilepsy drugs regularly. Patients do not take sleeping pills or psychotropic groups and willing to be included in this study by signing informed consent. The patient with use sleeping pills or psychotropic groups, epileptic seizures caused by structural abnormalities or lesions in the brain, and serious diseases, such as malignancy or other complications, were excluded.
ResultsIn this study, respondents were obtained from Neurology Poly RSUP Dr. Wahidin Sudirohusodo and his Network, Makassar RSUH, and Neurologist Physician. Assessment of sleep quality by using the Pittsburgh Sleep Quality Index (PSQI) questionnaire and the degree of insomnia using the Insomnia Severity Index (ISI) questionnaire. Respondents were focal and general epilepsy patients aged 18–55 years.
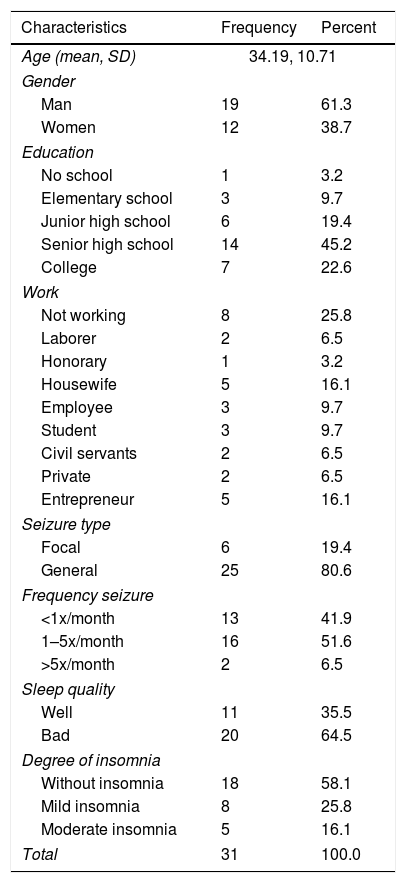
From the study, Table 1 shows 31 respondents who met the inclusion criteria and all were used as research subjects. Respondents consisted of 19 people (61.3%) men and 12 people (38.7%) women.
Characteristics of respondents.
| Characteristics | Frequency | Percent |
|---|---|---|
| Age (mean, SD) | 34.19, 10.71 | |
| Gender | ||
| Man | 19 | 61.3 |
| Women | 12 | 38.7 |
| Education | ||
| No school | 1 | 3.2 |
| Elementary school | 3 | 9.7 |
| Junior high school | 6 | 19.4 |
| Senior high school | 14 | 45.2 |
| College | 7 | 22.6 |
| Work | ||
| Not working | 8 | 25.8 |
| Laborer | 2 | 6.5 |
| Honorary | 1 | 3.2 |
| Housewife | 5 | 16.1 |
| Employee | 3 | 9.7 |
| Student | 3 | 9.7 |
| Civil servants | 2 | 6.5 |
| Private | 2 | 6.5 |
| Entrepreneur | 5 | 16.1 |
| Seizure type | ||
| Focal | 6 | 19.4 |
| General | 25 | 80.6 |
| Frequency seizure | ||
| <1x/month | 13 | 41.9 |
| 1–5x/month | 16 | 51.6 |
| >5x/month | 2 | 6.5 |
| Sleep quality | ||
| Well | 11 | 35.5 |
| Bad | 20 | 64.5 |
| Degree of insomnia | ||
| Without insomnia | 18 | 58.1 |
| Mild insomnia | 8 | 25.8 |
| Moderate insomnia | 5 | 16.1 |
| Total | 31 | 100.0 |
Based on the latest education level, one person (3.2%) did not take formal education, three people (9.7%) graduated from elementary school, six people (19.4%) graduated from junior high school, 14 people (45.2%) high school graduates, and seven people (22.6%) graduated from universities.
Based on the work, there were 8 people (25.8%) who did not work, 2 people (6.5%) as laborers, 1 person (3.2%) honorary, 5 people (16.1%) housewives, 3 people (9.7%) employees, 3 people (9.7%) students, 2 people (6.5%) civil servants, 2 people (6.5%) private workers, and 5 people (16.1%) self-employed workers.
Based on sleep quality and degree of insomnia, 11 sleep quality was good (35.5%), and 20 people had poor sleep quality (64.5%). Whereas for the degree of insomnia, there were 18 people without insomnia (18%), eight people with mild insomnia (25.8%), and insomnia being five people (16.1%).
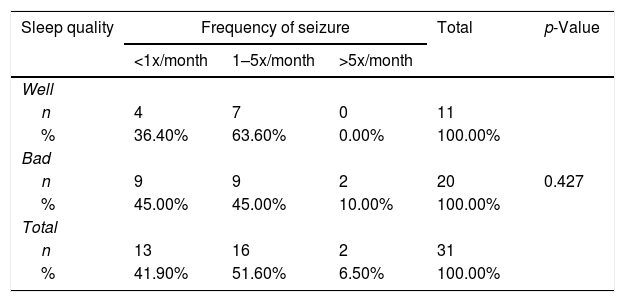
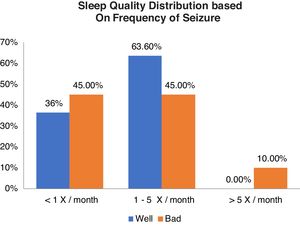
Relationship between sleep quality and generating frequencyFrom Table 2 shows good sleep quality as many as 11 respondents including 4 respondents (36.4%) with generation frequency <1x/month, 7 respondents (63.6%) with generation frequency 1–5x/month, and no experience >5x/month. While the poor sleep quality was 20 respondents including 9 respondents (45.0%) with generation frequency <1x/month, 9 respondents (45.0%) with trip frequency 1–5x/month, and 2 respondents (10.0%) with >5x/month. From the results of statistical tests obtained p value (0.427) >0.05, which means there is no relationship between the quality of sleep and frequency of generation (Graph 1).
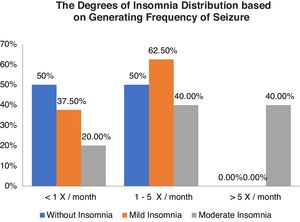
Table 3 shows the relationship between the degree of insomnia with a frequency of seizures, without insomnia as many as 18 respondents, including nine respondents each (50.0%) with a frequency of seizures <1x/month and 1–5x/month. The categories of mild insomnia were 8 respondents including 3 respondents (37.5%) with frequency of seizures <1x/month and 5 respondents (62.5%) with frequency of seizures 1-5x/month and moderate insomnia categories of 5 respondents including 1 respondent (20.0%) with generating frequency <1x/month and each of the 2 respondents (40.0%) with generation frequency 1–5x/month and >5x/month. The results of statistical tests obtained p-value (0.020) <0.05, which means that there is a relationship between the degree of insomnia and frequency of seizures (Graph 2).
Degree of insomnia distribution of frequency generation.
| Derajat insomnia | Frequency of seizure | Total | p-Value | ||
|---|---|---|---|---|---|
| <1x/month | 1–5x/month | >5x/month | |||
| Without insomnia | 0.020 | ||||
| n | 9 | 9 | 0 | 18 | |
| % | 50.00% | 50.00% | 0.00% | 100.00% | |
| Mild insomnia | |||||
| n | 3 | 5 | 0 | 8 | |
| % | 37.50% | 62.50% | 0.00% | 100.00% | |
| Moderate insomnia | |||||
| n | 1 | 2 | 2 | 5 | |
| % | 20.00% | 40.00% | 40.00% | 100.00% | |
| Total | |||||
| n | 13 | 16 | 2 | 31 | |
| % | 41.90% | 51.60% | 6.50% | 100.00% | |
Based on the results of the study found 31 respondents who met the inclusion criteria, and all of them were used as research subjects. From the interview results, the data obtained from 13 people (41.9%) did not experience seizures in the last one month, while 18 people (58.1%) experienced seizures with varying frequency, the highest frequency being 1–5x seizures. The results of the sleep quality score, as measured by the PSQI questionnaire, obtained the lowest score of 2 points, and the highest score was 18 points. While the results of the degree of insomnia score, as measured by the ISI questionnaire, obtained the lowest score of 1 point and the highest score of 21 points.
Sleep is a diverse and complex event, originating from several processes in the brain, which include several neural circuits that are interconnected with each other and some neurotransmitters that play a role in the process of sleep–wake up and influence each other.4,11
Normally brain activity occurs due to the transfer of signals from one neuron to another neuron. This displacement occurs between the terminal axons of a neuron with another dendrite neuron through the synapse. Synapse is an important area for electrolyte transfer and the secretion of neurotransmitters inside the presynaptic vesicles.1,2,13
The composition of electrolytes and neurotransmitters influence each other. It maintains the balance of ion gradients inside and outside the cell. Through the bond between neurotransmitters, their receptors, and the entry and exit of electrolytes through their canals. These activities will cause depolarization, hyperpolarization, and repolarization so that there is potential for excitation and inhibition of neuron cells. Excitation potential is projected by neuron cells in the cortex, which are then forwarded by axons, while interneuron cells function as inhibition.1,2,13
Areas that play a role in the waking process include Ascending Reticular Activating System (ARAS), Nucleus Solitarius Tract, Locus Coeruleus, Raphe Nucleus, Nucleus Laterodorsal Tegmentum and Pedunculopontin Tegementum (LTD/PPT nuclei), Mesolimbic System, Nucleus Tuberomamilaris (TMN), Perifornical Nucleus, Suprachiasmatic Nucleus (SCN), Hypothalamic Preoptic Area, Ventrolateral Preoptic Nucleus (VLPO nuclei), Preoptic Median Nucleus (MPN), Subparaventricular Zone, Dorsomedial Nucleus, Frontalis Base (innominate substance), Meynert nucleus basalis, Amygdala-related neurons. nucleus accumbens and ventral putamen, limbic system, and thalamus.4,14
The neurotransmitters that play a role in the wakefulness process are such as catecholamines, acetylcholine, serotonin (5-HT, 5-hydroxytryptamine), dopamine, histamine, glutamate, GABA, G alanine, glycine, pituitary hormones and other related components, hypocretin (orexin), and Delta sleep-inducing peptide (DSIP). The other neurotransmitters that also play a role are prostaglandin, oleamide, cannabinoid, adenosine, uridine, and nitrite oxide.4,11,16
The wake-up cycle includes complex internal neural circuits. In adults, it is divided into 5 phases, namely phases 1–4 called non-rapid eye movement sleep (NREM) and the fifth phase called rapid eye movement (REM). Phases 1 and 2 are called NREM light, while phases 3 and 4 are called deep NREM, which are seen as delta waves or slow-wave sleep (SWS).6–8,11,16
Epileptic seizures occur when the excitation and inhibition process is not balanced, causing hyperexcitability. Internal and external factors influence the imbalance of inhibition and excitation processes. An epileptiform release can be activated or inhibited depending on the stage of sleep. Epileptiform release tends to spread during NREM sleep because it is more synchronized, whereas, during REM sleep, it appears asynchronous wave release. Reduction of sleep time is associated with seizures and epileptiform wave release in epilepsy patients.15
From the results of this study, using the chi-square test that assesses the relationship of sleep quality to generation frequency with the PSQI indicator obtained a value of p=0.427 (>0.05), which means there is no relationship between sleep quality and generation frequency. In contrast, the relationship of insomnia to generation frequency with the ISI indicator p-value=0.020 (<0.05), which means that there is a relationship between the degree of insomnia on the frequency of the trip.
Some case–control studies found that poor sleep quality, insomnia, and excessive daytime sleepiness were two to three times more common in people with epilepsy than in healthy controls. Comorbid sleep disorders can also be found in epilepsy patients, in the form of excessive sleepiness during the day, sleep breathing disorders (obstructive sleep apnea), insomnia, drug effects or substance abuse, fear of seizures during sleep.5,10,13
Lee et al.15 illustrates that sleep disorders especially insomnia are more common in people with epilepsy, the relationship between clinical and demographic characteristics of epilepsy patients with the results of the PSQI test does not prove there is a statistically relevant influence.
ConclusionFrom the results of this study, it can be concluded that sleep quality is not related to the frequency of generation, while the degree of insomnia is related to the frequency of seizures, where the frequency of seizures in patients with moderate insomnia is higher than those without insomnia.
Conflict of interestThe authors declare no conflict of interest.
Peer-review under responsibility of the scientific committee of the Technology Enhanced Medical Education International Conference (THEME 2019). Full-text and the content of it is under responsibility of authors of the article.