In non-valvular atrial fibrillation (NVAF) patients at risk of stroke, anticoagulant drugs are less likely to be received by older patients than younger patients. In this study, an attempt is made to discover whether the reasons reported by physicians for denying anticoagulant drugs prescription differ between older and younger atrial fibrillation patients.
Materials and methodsA retrospective, cross-sectional, multicentre study was conducted from October 2014 to July 2015. The study comprised patients aged ≥18 years diagnosed with NVAF, with a moderate to high stroke risk (CHADS2 score ≥2). Patients were stratified according to age (<80 and ≥80 years).
ResultsA total of 1309 NVAF patients were evaluated, of whom 40.1% were ≥80 years old. Older patients were predominantly women with higher mean time since diagnosis of AF, with a higher rate of permanent NVAF, and with higher thromboembolic risk. In patients for whom physicians decided not to prescribe any anticoagulant agents, the following reasons were significantly more frequent in patients aged ≥80 years compared to younger patients: cognitive impairment, perceived high bleeding risk, falls, difficult access to monitoring, non-neoplastic terminal illness, and perceived low thromboembolic risk. Uncontrolled hypertension was a significantly more frequent reason for non-prescription of anticoagulant agents in patients aged <80 year.
ConclusionsOctogenarian patients with NVAF and a moderate to high risk of stroke had a different as regards reasons for not being prescribed anticoagulant agents, which should be taken into account in order to improve.
Los pacientes con fibrilación auricular no valvular (FANV) y riesgo de accidente cerebrovascular de mayor edad tienen menor probabilidad de recibir tratamiento anticoagulante que los de menor edad. En este estudio tratamos de identificar si las razones reportadas de los médicos para negar la prescripción de medicamentos anticoagulantes difieren entre los pacientes con fibrilación auricular de menor y mayor edad.
Materiales y métodosEstudio retrospectivo, transversal, multicéntrico realizado entre octubre de 2014 y julio de 2015. El estudio incluyó pacientes ≥18 años, diagnosticados con FANV, y riesgo de accidente cerebrovascular de moderado a alto (puntuación CHADS2≥2). Los pacientes fueron estratificados según edad (<80 y ≥80 años).
ResultadosSe evaluaron 1.309 pacientes con FANV (el 40,1% era ≥80 años). Los pacientes de mayor edad eran predominantemente mujeres con un mayor tiempo medio desde el diagnóstico de FA, mayor tasa de FANV permanente y mayor riesgo tromboembólico. Las razones significativamente más frecuentes para no prescribir tratamiento anticoagulante en pacientes ≥80 años, en comparación con pacientes <80 años, fueron: deterioro cognitivo, riesgo percibido de sangrado elevado, caídas, difícil acceso a la monitorización, enfermedad terminal no neoplásica y riesgo tromboembólico percibido bajo. La hipertensión no controlada fue un motivo significativamente más frecuente para la no prescripción de tratamiento anticoagulante en pacientes <80 años.
ConclusionesLos pacientes octogenarios con FANV y riesgo de accidente cerebrovascular moderado a alto presentan un perfil diferencial con respecto a los motivos para la no prescripción de tratamiento anticoagulante y que deben tenerse en cuenta para mejorar.
Atrial fibrillation (AF) is the most common rhythm abnormality and its prevalence increases with age.1 AF is associated with significant morbidity and mortality, especially in older patients.2 In addition, AF diagnosis is associated with serious health implications, including a 5-fold increase in stroke risk. About 1.1 million European inhabitants suffered a stroke each year, but due to the ageing of the European population, the proportion of stroke is expected to dramatically increase in coming years (1.5 million European people by 2025).3 For this reason, prevention of stroke is an important goal in older AF patients.4
The most recent guidelines for clinical management of AF recommend that all non-valvular AF (NVAF) patients with a moderate to high risk of stroke (CHADS2 ≥2 or CHA2DS2-VASc ≥2) should receive anticoagulant treatment with oral anticoagulants (OACs) unless contraindicated, with direct OACs (DOACs) the preferred option over vitamin K antagonists.5 However, a systematic review of 54 real-world studies shows underuse of OACs, even in patients with an elevated risk of stroke.6 Physicians reasons given for not prescribing are various. So, age, degree of education, and years of experience had been reported to have with significant positive influence on awareness to use oral anticoagulantion.7 In contrast, high DOAC cost had been reported as a reason for not prescribing them compared to other anticoagulants such as warfarin.8
Age is an independent predictor of stroke in AF patients, but also of bleeding risk. Moreover, AF is a more complex condition in older subjects due to their frailty, high number of comorbidities (including cardiovascular and kidney diseases), cognitive disorders, falls and polypharmacy.4 Likely for all of these reasons, underuse of anticoagulation therapy is higher in older patients.9 The possibility of using new OACs (DOACs) has been reported to be associated with an increase in the use of anticoagulants in older people.10
We recently reported that approximately 1 in 5 older Spanish patients (mean age 75 years) diagnosed with NVAF and with a moderate to high associated risk of stroke do not receive OACs in clinical practice.11 These patients are characterized by comorbidities and a high prevalence of modifiable risk factors, such as uncontrolled hypertension, and concomitant drugs that increase the bleeding risk. Following this line of research, the main objective of the present study was to evaluate whether physicians’ reported reasons for denying anticoagulant drugs prescription differ between older and younger (<80 and ≥80 years) atrial fibrillation patients with NVAF and CHADS2 ≥2 who were not being treated with OACs. Our hypothesis was that the reasons for not prescribing anticoagulants in the real world would differ according to patient age.
Material and methodsThis was an observational, cross-sectional, retrospective, nationwide, multicentre study conducted in Spain from October 2014 to July 2015. The methods of the study as well as the selection of patients and researchers were reported previously.11 Briefly, 230 primary care physicians (PCPs) throughout Spain participated by including patients and collecting data (from 15 of the 17 Spanish Autonomous Communities). The study population was 1504 patients (≥18 years) with a diagnosis of NVAF (according to ESC Guidelines) documented in their medical records and a moderate to high risk of stroke (CHADS2 ≥2), who were not being treated with OACs at the time of the study. Of these patients, 1309 (87.1%) were considered evaluable for the present analysis. The main reasons for exclusion from the analysis were data being of insufficient quality to evaluate the study objectives (n=122, 8.1%) and non-fulfilment of the selection criteria (n=51, 3.4%). Other reasons included protocol deviations (n=21, 1.4%). Pregnant and breastfeeding women were also excluded.
All study participants provided written informed consent prior to study enrolment. Sociodemographic, AF clinical history and pharmacological treatment data were collected from medical records. The thromboembolic risk was assessed using CHADS2 and CHA2DS2-VASc, and the bleeding risk using the HAS-BLED scale.12–14 Reported reasons for decisions about clinical management of stroke prevention were also collected. Physicians were free to choose more than one options to support their decision.
Cognitive impairment/dementia was assessed using both the investigator's assessment question (IAQ) Does this patient have dementia? (yes or no), and with the Global Deterioration Scale (GDS).15 The GDS scale has 7 stages and the overall score ranges from 1 (no dementia) to 7 (late-stage dementia). Disability/dependence in activities of daily living (ADL) was assessed using the Barthel Index.16 The Barthel Index has 10 items and the overall score ranges from 0 to 100. Scores were classified as follows: 100, no dependence; 91–99, mild dependence; 61–90, moderate dependence; 21–60, severe dependence; and 0–20, total dependence.
In addition, each participating PCP provided summary information regarding the population covered by each family practice and healthcare centre, and the total number of patients attended to by the centre with (i) AF, (ii) NVAF and (iii) NVAF with a moderate to high thromboembolic risk (CHADS2 ≥2) who were not being treated with OACs (overall, by gender and according to age categories). The study was reviewed and approved by the ethics committee at University Hospital Ramón y Cajal (Madrid, Spain).
For the purpose of this subanalysis, patients were stratified according to their age at baseline (<80 and ≥80 years).
Statistical analysisFor the descriptive analysis, quantitative variables were described with measures of central tendency and dispersion (mean, standard deviation, median and range) and qualitative variables were described as absolute (n) and relative (%) frequencies. Differences between patients according to age were analyzed by chi-square test, Fisher's exact test or likelihood-ratio test (as appropriate) for categorical variables, and using the t-test or Mann–Whitney test for quantitative variables.
Statistical analyses were performed using the IBM SPSS® statistical package for Windows (version 19.0; IBM Corp., Armonk, NY).
ResultsA total of 1309 NVAF patients with CHADS2 ≥2 who were not being treated with OACs were evaluated, of whom 526 (40.1%) were ≥80 years old. The clinical decision for stroke prevention was antiplatelet therapy in 1005 patients (75.6%), heparins in 64 patients (4.8%) and no treatment in the 240 remaining patients (19.6%)
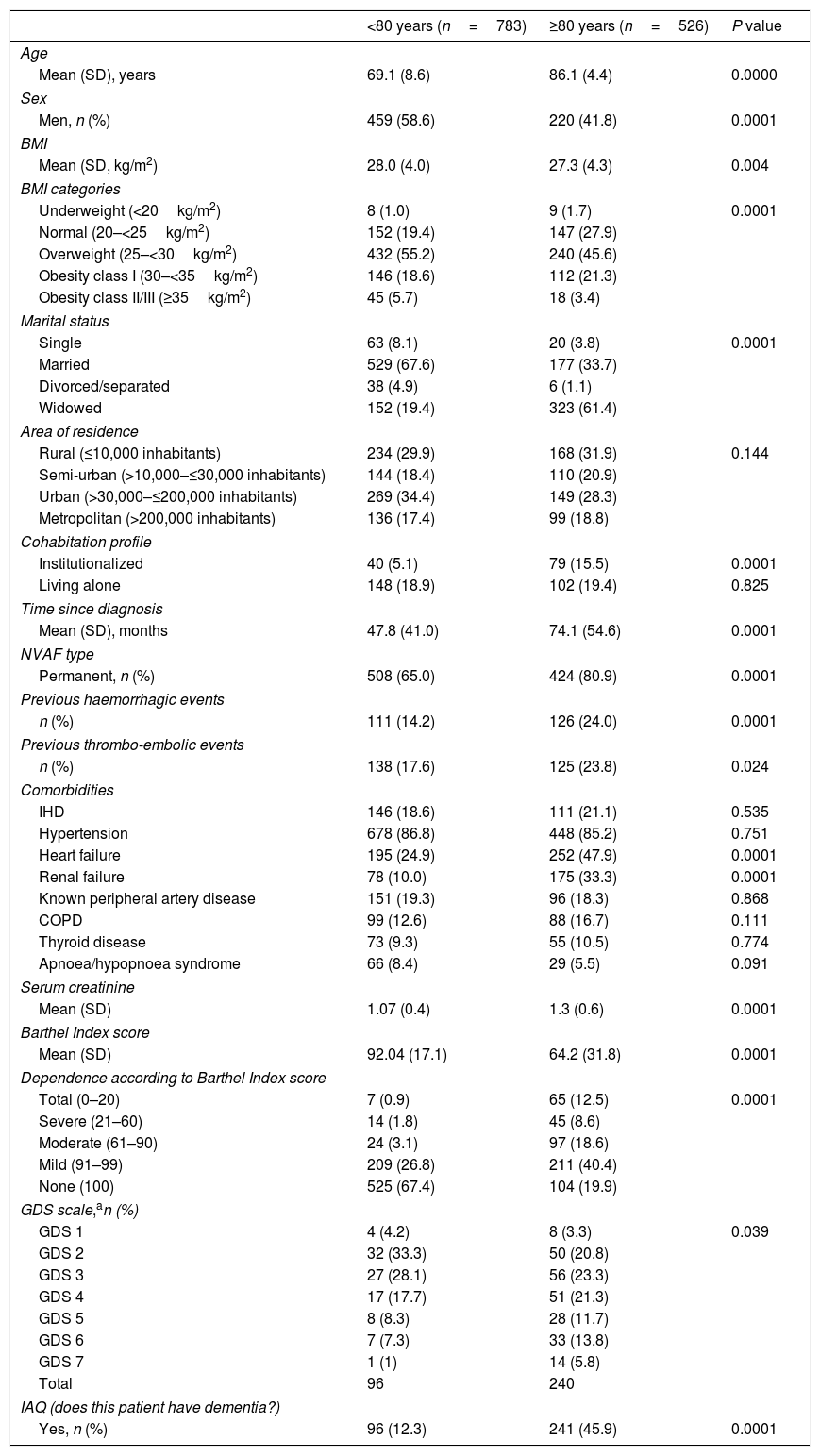
Table 1 lists the main demographic and clinical characteristics of the study sample according to age categories. Older patients were predominantly women, widowed, with lower BMI values, high rates of institutionalization, higher rates of heart failure and chronic renal failure, and poor renal function.
Demographic and clinical characteristics according to age.
| <80 years (n=783) | ≥80 years (n=526) | P value | |
|---|---|---|---|
| Age | |||
| Mean (SD), years | 69.1 (8.6) | 86.1 (4.4) | 0.0000 |
| Sex | |||
| Men, n (%) | 459 (58.6) | 220 (41.8) | 0.0001 |
| BMI | |||
| Mean (SD, kg/m2) | 28.0 (4.0) | 27.3 (4.3) | 0.004 |
| BMI categories | |||
| Underweight (<20kg/m2) | 8 (1.0) | 9 (1.7) | 0.0001 |
| Normal (20–<25kg/m2) | 152 (19.4) | 147 (27.9) | |
| Overweight (25–<30kg/m2) | 432 (55.2) | 240 (45.6) | |
| Obesity class I (30–<35kg/m2) | 146 (18.6) | 112 (21.3) | |
| Obesity class II/III (≥35kg/m2) | 45 (5.7) | 18 (3.4) | |
| Marital status | |||
| Single | 63 (8.1) | 20 (3.8) | 0.0001 |
| Married | 529 (67.6) | 177 (33.7) | |
| Divorced/separated | 38 (4.9) | 6 (1.1) | |
| Widowed | 152 (19.4) | 323 (61.4) | |
| Area of residence | |||
| Rural (≤10,000 inhabitants) | 234 (29.9) | 168 (31.9) | 0.144 |
| Semi-urban (>10,000–≤30,000 inhabitants) | 144 (18.4) | 110 (20.9) | |
| Urban (>30,000–≤200,000 inhabitants) | 269 (34.4) | 149 (28.3) | |
| Metropolitan (>200,000 inhabitants) | 136 (17.4) | 99 (18.8) | |
| Cohabitation profile | |||
| Institutionalized | 40 (5.1) | 79 (15.5) | 0.0001 |
| Living alone | 148 (18.9) | 102 (19.4) | 0.825 |
| Time since diagnosis | |||
| Mean (SD), months | 47.8 (41.0) | 74.1 (54.6) | 0.0001 |
| NVAF type | |||
| Permanent, n (%) | 508 (65.0) | 424 (80.9) | 0.0001 |
| Previous haemorrhagic events | |||
| n (%) | 111 (14.2) | 126 (24.0) | 0.0001 |
| Previous thrombo-embolic events | |||
| n (%) | 138 (17.6) | 125 (23.8) | 0.024 |
| Comorbidities | |||
| IHD | 146 (18.6) | 111 (21.1) | 0.535 |
| Hypertension | 678 (86.8) | 448 (85.2) | 0.751 |
| Heart failure | 195 (24.9) | 252 (47.9) | 0.0001 |
| Renal failure | 78 (10.0) | 175 (33.3) | 0.0001 |
| Known peripheral artery disease | 151 (19.3) | 96 (18.3) | 0.868 |
| COPD | 99 (12.6) | 88 (16.7) | 0.111 |
| Thyroid disease | 73 (9.3) | 55 (10.5) | 0.774 |
| Apnoea/hypopnoea syndrome | 66 (8.4) | 29 (5.5) | 0.091 |
| Serum creatinine | |||
| Mean (SD) | 1.07 (0.4) | 1.3 (0.6) | 0.0001 |
| Barthel Index score | |||
| Mean (SD) | 92.04 (17.1) | 64.2 (31.8) | 0.0001 |
| Dependence according to Barthel Index score | |||
| Total (0–20) | 7 (0.9) | 65 (12.5) | 0.0001 |
| Severe (21–60) | 14 (1.8) | 45 (8.6) | |
| Moderate (61–90) | 24 (3.1) | 97 (18.6) | |
| Mild (91–99) | 209 (26.8) | 211 (40.4) | |
| None (100) | 525 (67.4) | 104 (19.9) | |
| GDS scale,an (%) | |||
| GDS 1 | 4 (4.2) | 8 (3.3) | 0.039 |
| GDS 2 | 32 (33.3) | 50 (20.8) | |
| GDS 3 | 27 (28.1) | 56 (23.3) | |
| GDS 4 | 17 (17.7) | 51 (21.3) | |
| GDS 5 | 8 (8.3) | 28 (11.7) | |
| GDS 6 | 7 (7.3) | 33 (13.8) | |
| GDS 7 | 1 (1) | 14 (5.8) | |
| Total | 96 | 240 | |
| IAQ (does this patient have dementia?) | |||
| Yes, n (%) | 96 (12.3) | 241 (45.9) | 0.0001 |
Data are n (%) except where otherwise indicated.
BMI=body mass index; COPD=chronic obstructive pulmonary disease; GDS=Global Deterioration Scale; IAQ=investigator's assessment question; IHD=ischaemic heart disease; NVAF=non-valvular atrial fibrillation; SD=standard deviation.
The mean (SD) time since diagnosis of AF was 58.4 (48.6) months, and almost three quarters (71.4%) of patients had permanent NVAF. Patients aged ≥80 years had a higher mean time since diagnosis of AF and a higher rate of permanent NVAF (Table 1). They also had higher rates of previous thromboembolic and bleeding events (Table 1).
Functional and cognitive statusThe mean (SD) Barthel Index score was 80.9 (27.7) points. Mean Barthel Index score was significantly lower in older patients (64 vs. 92 points) (Table 1). Based on the IAQ, 25.7% of patients were identified as having dementia, with a higher percentage in the older age group (45.9% vs. 12.3%). When applying the GDS scale there were also higher rates of poor cognitive status in patients aged ≥80 years (Table 1).
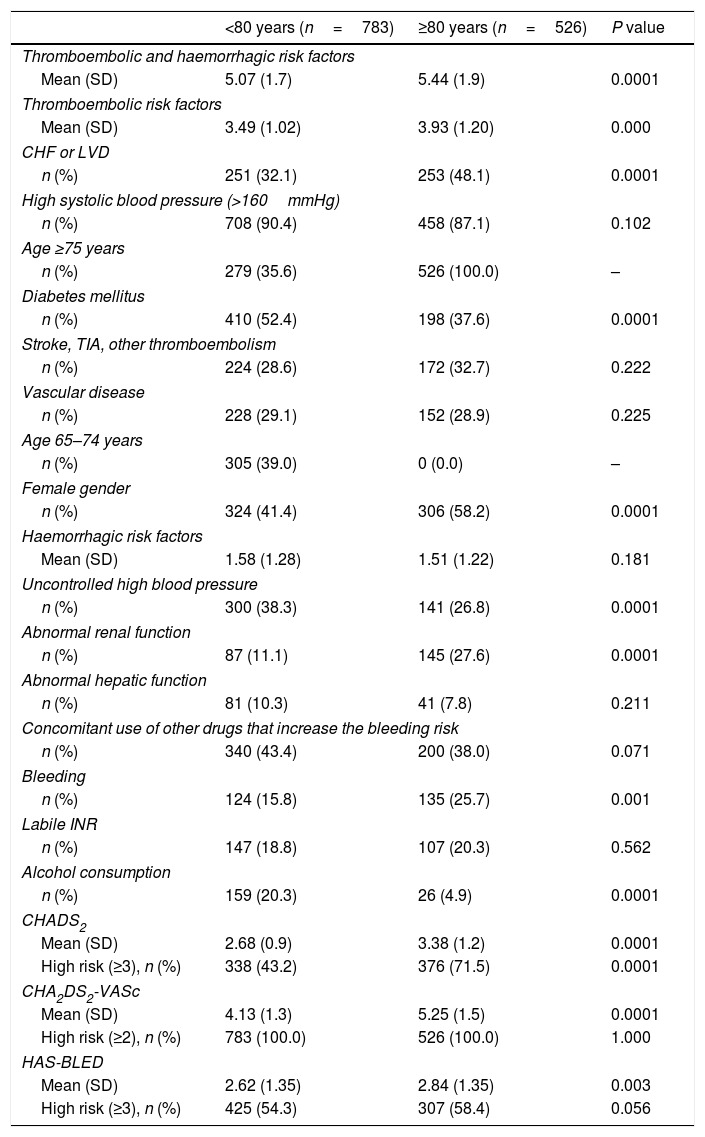
Thromboembolic and haemorrhagic riskThe thromboembolic risk was higher in older patients, according to CHADS2 and CHA2DS2-VASc (Table 2). In addition, the rate of congestive heart failure was significantly higher in older patients, but the rate of diabetes mellitus was lower.
Haemorrhagic and thromboembolic risk factors and risk scores (CHADS2, CHA2DS2-VASc and HAS-BLED) according to age.
| <80 years (n=783) | ≥80 years (n=526) | P value | |
|---|---|---|---|
| Thromboembolic and haemorrhagic risk factors | |||
| Mean (SD) | 5.07 (1.7) | 5.44 (1.9) | 0.0001 |
| Thromboembolic risk factors | |||
| Mean (SD) | 3.49 (1.02) | 3.93 (1.20) | 0.000 |
| CHF or LVD | |||
| n (%) | 251 (32.1) | 253 (48.1) | 0.0001 |
| High systolic blood pressure (>160mmHg) | |||
| n (%) | 708 (90.4) | 458 (87.1) | 0.102 |
| Age ≥75 years | |||
| n (%) | 279 (35.6) | 526 (100.0) | – |
| Diabetes mellitus | |||
| n (%) | 410 (52.4) | 198 (37.6) | 0.0001 |
| Stroke, TIA, other thromboembolism | |||
| n (%) | 224 (28.6) | 172 (32.7) | 0.222 |
| Vascular disease | |||
| n (%) | 228 (29.1) | 152 (28.9) | 0.225 |
| Age 65–74 years | |||
| n (%) | 305 (39.0) | 0 (0.0) | – |
| Female gender | |||
| n (%) | 324 (41.4) | 306 (58.2) | 0.0001 |
| Haemorrhagic risk factors | |||
| Mean (SD) | 1.58 (1.28) | 1.51 (1.22) | 0.181 |
| Uncontrolled high blood pressure | |||
| n (%) | 300 (38.3) | 141 (26.8) | 0.0001 |
| Abnormal renal function | |||
| n (%) | 87 (11.1) | 145 (27.6) | 0.0001 |
| Abnormal hepatic function | |||
| n (%) | 81 (10.3) | 41 (7.8) | 0.211 |
| Concomitant use of other drugs that increase the bleeding risk | |||
| n (%) | 340 (43.4) | 200 (38.0) | 0.071 |
| Bleeding | |||
| n (%) | 124 (15.8) | 135 (25.7) | 0.001 |
| Labile INR | |||
| n (%) | 147 (18.8) | 107 (20.3) | 0.562 |
| Alcohol consumption | |||
| n (%) | 159 (20.3) | 26 (4.9) | 0.0001 |
| CHADS2 | |||
| Mean (SD) | 2.68 (0.9) | 3.38 (1.2) | 0.0001 |
| High risk (≥3), n (%) | 338 (43.2) | 376 (71.5) | 0.0001 |
| CHA2DS2-VASc | |||
| Mean (SD) | 4.13 (1.3) | 5.25 (1.5) | 0.0001 |
| High risk (≥2), n (%) | 783 (100.0) | 526 (100.0) | 1.000 |
| HAS-BLED | |||
| Mean (SD) | 2.62 (1.35) | 2.84 (1.35) | 0.003 |
| High risk (≥3), n (%) | 425 (54.3) | 307 (58.4) | 0.056 |
CHF=congestive heart failure; INR=international normalized ratio; LVD=left ventricular dysfunction; SD=standard deviation; TIA=transient ischaemic attack.
Based on HAS-BLED data, the bleeding risk was lower in younger patients (Table 2). Patients aged ≥80 years had lower rates of uncontrolled blood pressure and alcohol consumption, and higher rates of abnormal renal function and previous bleeding of any cause.
Concomitant treatmentsBesides antiplatelet drugs, the most common pharmacological treatment in the study population was anti-hypertensive agents (85.2%), followed by drugs for gastric protection (76.7%), drugs for heart rate control (57.1%), antidiabetic drugs (34.2%), antiarrhythmic drugs (28.4%) and non-steroidal anti-inflammatory drugs (NSAIDs) (27.6%). Patients aged ≥80 years had lower rates of antiarrhythmic and antidiabetic drug use, and higher rates of gastric protection drug use (Supplementary Table 1).
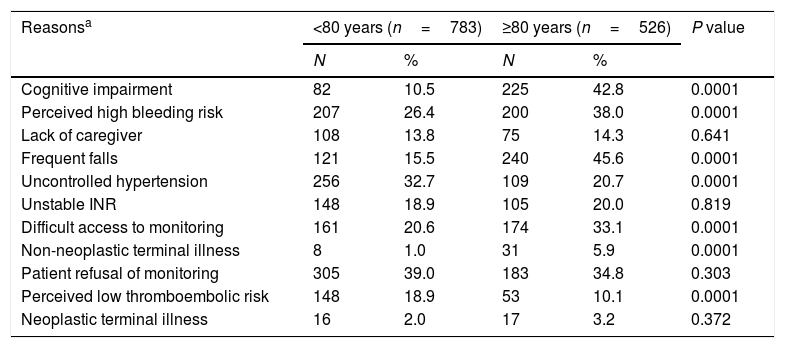
Reasons for clinical management decisions at diagnosis of NVAFAs previously published,11 the main reasons that PCPs gave for their decision not to prescribe OACs in the overall study population were perceived high bleeding risk (31.1%), recurrent falls (27.6%), uncontrolled hypertension (27.9%), difficult access to monitoring (25.6%) and cognitive impairment (23.4%). Table 3 shows the main reasons for not prescribing OACs according to age group (<80 years vs ≥80 years). In patients for whom physicians decided not to prescribe anticoagulant agents, the following reasons were significantly more frequent in patients aged ≥80 years compared to younger patients: cognitive impairment, perceived high bleeding risk, recurrent falls, difficult access to monitoring, non-neoplastic terminal illness and perceived low thromboembolic risk. Uncontrolled hypertension was a more frequent reason for non-prescription of anticoagulant agents in patients aged <80 years.
Reasons for clinical management decisions.
| Reasonsa | <80 years (n=783) | ≥80 years (n=526) | P value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Cognitive impairment | 82 | 10.5 | 225 | 42.8 | 0.0001 |
| Perceived high bleeding risk | 207 | 26.4 | 200 | 38.0 | 0.0001 |
| Lack of caregiver | 108 | 13.8 | 75 | 14.3 | 0.641 |
| Frequent falls | 121 | 15.5 | 240 | 45.6 | 0.0001 |
| Uncontrolled hypertension | 256 | 32.7 | 109 | 20.7 | 0.0001 |
| Unstable INR | 148 | 18.9 | 105 | 20.0 | 0.819 |
| Difficult access to monitoring | 161 | 20.6 | 174 | 33.1 | 0.0001 |
| Non-neoplastic terminal illness | 8 | 1.0 | 31 | 5.9 | 0.0001 |
| Patient refusal of monitoring | 305 | 39.0 | 183 | 34.8 | 0.303 |
| Perceived low thromboembolic risk | 148 | 18.9 | 53 | 10.1 | 0.0001 |
| Neoplastic terminal illness | 16 | 2.0 | 17 | 3.2 | 0.372 |
Although the number of octogenarians with AF who receive anticoagulants in Europe and Canada has increased in recent years and now exceeds 70%,17,18 and with the knowledge that vitamin K antagonist anticoagulation quality is similar in octogenarians and in younger patients,19 we decided to explore the characteristics of octogenarians not receiving OACs. The main finding of the present study is that the profile of patients with NVAF and CHADS2 ≥2 who were not receiving OACs in primary care in Spain in 2014–2015 differed according the age, as did the physicians’ reported reasons for non-prescription of OACs.
Older patients were predominantly women, widowed, with lower BMI values, high rates of institutionalization, higher rates of heart failure, and poor cognitive and functional status. Also, as it is shown in Table 2 and although it was not an objective of this work, our results confirm that octogenarians with NVAF not receiving OACs had a high risk of stroke and bleeding and frequently had renal disease.19
Among patients ≥80 years old, previous stroke and heart failure were significantly associated with OAC use.17 In the present study, older patients not receiving OACs had a higher rate of previous congestive heart failure, but there were no significant differences according to age for previous stroke or other thromboembolism.
A number of factors appear to influence the decision not to prescribe OACs for octogenarians with AF, which is in line with previously published studies.18,19 Our findings are similar to data found in other studies. Recurrent falls (47%), cognitive impairment (22.6%) and advanced age (16.4%) were the main reasons reported by physicians for not prescribing anticoagulants in older patients (mean age 87 years) in a nursing home setting in France.20 In the Spanish AFABE study,21 conducted in 2012, the most common reasons for not administering or for discontinuing OACs in patients with AF were cognitive impairment (15.1%), a too-high risk of haemorrhage (14.3%) and patient refusal or preference (13.6%). Females are less likely to receive anticoagulants than males,22 so it is very important to consider that in very old AF patient samples there is a predominance of women.
Despite the age-associated increase in bleeding risk, there is no recommendation to avoid OAC treatment.23 Rather than not giving the right anticoagulant treatment, high risk scores of bleeding should be used to intensify attention on potentially modifiable risk factors, such as uncontrolled blood pressure and/or possible drug interactions.
It is important to emphasize that one of the most frequent reason for non-prescription of OACs, recurrent falls can be considered a subjective reason for no-prescription, in the absence of clear scientific evidence.4 In fact, it has been estimated that a patient would need to fall approximately 300 times in 1 year for the increased risk of intracranial haemorrhage to outweigh the benefits of anticoagulation in preventing thromboembolism.24
Because of its importance we explore the presence of any cognitive deficit with a direct question and with the GDS classification. The frequency of cognitive impairment increases with age, although on its own it should not be a reason for non-prescription of anticoagulants, except perhaps in patients with dementia-related behaviour or advanced dementia.4 However, it was recently reported that discontinuing warfarin after diagnosis of dementia is associated with significant increases in stroke and mortality.25 Although the doctors who participated in the present study did not express it, we think that the misguided feeling that antiplatelet therapy is safe may be a reason for not giving anticoagulants to very old patients, despite its inefficiency.
The main strengths of our real-world study are inclusion of a meaningful number of participating centres from across the whole of Spain and inclusion of all patients in each centre who met the selection criteria, which ensured that our sample was representative. The daily practices and opinions of physicians caring for old people were reported, rather than comparisons with published recommendations.
Our study has some limitations. Because the study was observational and cross-sectional, it was impossible to evaluate causal relationships between thromboembolic events and treatments administered. Another limitation was the inability to individually check the NVAF diagnosis for each patient included in the study. Also is important to o keep in mind that the study only collected the reason but not if it was justified not to receive oral anticoagulation. Finally, some variables such as non-neoplastic terminal disease or frequent falls were not specified and were left to the discretion of the responding physician.
ConclusionsIn conclusion, even considering the high thromboembolic risk, there are several reasons for non-prescription of anticoagulants. Some such as haemorrhagic risk may be overestimated whereas others are very controversial, such as mild to moderate cognitive impairment and falls. Age by itself should never be a contraindication, although in very old people (≥80 years old) some of the most common reasons for not receiving anticoagulation are also present. Integrated care may improve the approach for elderly AF patients.
FundingThis work was sponsored by Pfizer and supported by the Alliance BMS-Pfizer.
Conflicts of interestDA and SFC are Pfizer employees. FF and JP have no conflicts of interest.