Pluripotent stem cells and spermatogonial stem cells are stem cell types specialized for reconstitution of germ cell development in vitro, as they are capable of differentiating into gametes. Reconstitution of germ cell development, termed in vitro gametogenesis, will provide an experimental platform for a better understanding of germ cell development, as well as an alternative source of artificial gametes for reproduction, with the potential to treat and cure infertility. Since germ cells are especialized cells that will pass all genetic information into the next generation, both the in vitro culture system and its derived-cells from stem cells must be carefully evaluated. In this review, we summarize the progress in in vitro gametogenesis, most of which has been made using mouse models, as well as the future challenges in this field.
Las células madre pluripotentes y las espermatogonias son tipos de células madre especializadas en la reconstitución del desarrollo de las células germinales in vitro, ya que son capaces de diferenciarse en gametos. La reconstitución del desarrollo de las células germinales, denominada gametogénesis in vitro, proporcionará una plataforma experimental para comprender mejor el desarrollo de las células germinales, así como una fuente alternativa de gametos artificiales para la reproducción, con el potencial de tratar y curar la infertilidad. Dado que las células germinales son células especializadas que transmitirán toda la información genética a la siguiente generación, tanto el sistema de cultivo in vitro como sus células derivadas a partir de células madre deben ser cuidadosamente evaluados. En esta revisión, resumimos los avances en la gametogénesis in vitro, la mayoría de los cuales se han realizado utilizando modelos de ratón, así como los retos futuros en este campo.
World Health Organization defined that the infertility is a disease of the reproductive system caused by the failure to achieve a clinical pregnancy after 1 year. Primary infertility is a type of infertility in a couple who have never had a child. Secondary infertility is a type of infertility that is the failure to conceive following a previous pregnancy.
Male factors are the cause of 25% of infertility cases, mainly due to sperm morphological and functional disorders, including hereditary diseases, precocious puberty and structural problems such as testicular blockage; genital damage or injury leading to sperm dysfunction, and environmental and psychological factors. Female factors include ovulation dysfunction, endometritis, abnormal uterus or fallopian tube, primary ovarian insufficiency and pelvic adhesions (Wang et al., 2019). Treatment for infertility varies from pharmacologic treatment to assisted reproductive technology (ART), depending on the cause and patient characteristics. Therapy for male infertility includes lifestyle improvements, medications, supplemments or antioxidants, surgery, and sperm regeneration. The primary therapy for female infertility is hormone medication, supplemments or antioxidants, surgery or an ART procedure, including intrauterine insemination (IUI), in vitro fertilization-embryo transfer (IVF), and intracytoplasmic sperm injection (ICSI).
In recent years, stem cells have garnered significant attention in the field of infertility (Vassena et al., 2015). Stem cells are cells with different origin and potency that can divide into various other cells for repair, development and regeneration. Studies of experimental models have shown that treating infertility with stem cell therapy is gaining acceptance (Somigliana et al., 2016). A series of female and male infertility studies using stem cells from different sources was recently launched. Pre-clinical studies on sexual infertility-related diseases have suggested new directions to consider for the treatment of infertility (Vanni et al., 2017). Studies using experimental models have revealed the power of stem cell therapy for treating infertility and verified these results (Brunauer et al., 2017; Volarevic et al., 2014). In this review, we will summarize the current knowledge regarding the potential use of stem cells derived gametes in mouse and human models for treating infertility related diseases in the future.
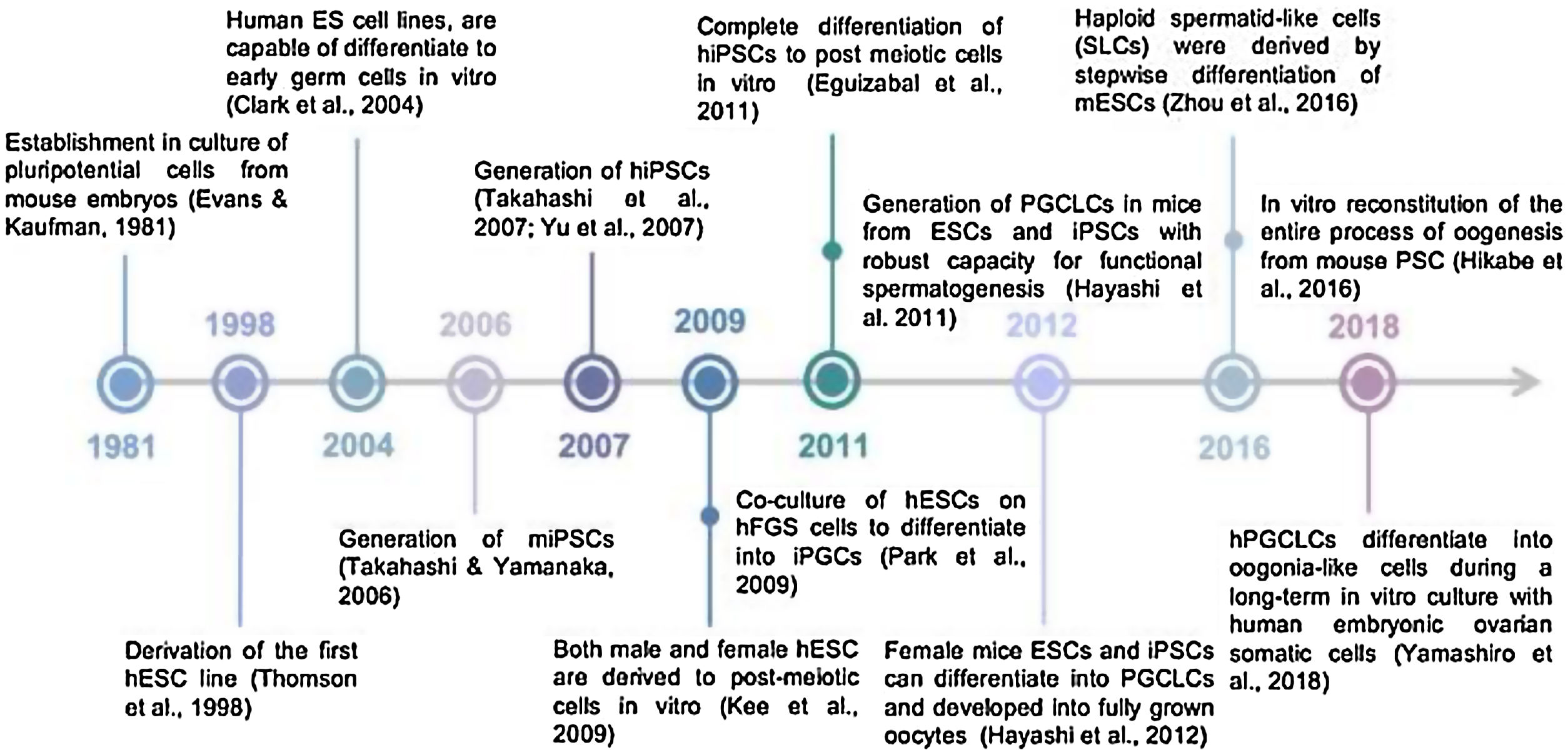
Historical timeline of pluripotent stem cell-derived germ cellsForty years ago, Evans & Kauffman discovered for the very first time the derivation of embryonic stem cells from early mouse embryos (Evans and Kaufman, 1981) (Fig. 1). Embryonic stem (ES) cells are pluripotent cells isolated from an early embryo and grown as a cell line in tissue culture. Their discovery came from the conjunction of studies in human pathology, mouse genetics, early mouse embryo development, cell surface immunology and tissue culture. ES cells provided a crucial tool for manipulating mouse embryos to study mouse genetics, development and physiology. They have not only revolutionized experimental mammalian genetics but, with the advent of equivalent human ES cells, have now opened new vistas for regenerative medicine.
Later, the detailed study of the biology of mouse stem cells led to the discovery, in 1998, of a method to derive stem cells from human embryos and grow the cells in the laboratory. These cells are called human embryonic stem cells (Fig. 1). The embryos used in these studies were created for reproductive purposes through in vitro fertilization procedures. When they were no longer needed for that purpose, they were donated for research with the informed consent of the donor (Thomson et al., 1998).
In 2006, the PSC field was revolutionized again by the generation of induced PSC (iPSC), a technology pioneered by Prof. Shinya Yamanaka in Japan. His research team demonstrated that forced expression of four transcription factors (Pou5f1, Sox2, Klf4, and c-Myc) could reprogram adult mouse cells into a pluripotent state remarkably similar to ESCs (Takahashi and Yamanaka, 2006) (Fig. 1). Less than one year later, the same technology was used for the generation of human iPSCs (hiPSCs) (Takahashi et al., 2007) (Fig. 1). This provides an alternative source of human pluripotent stem cells (hPSCs) without the need to use human embryos, thus relieving some of the ethical concerns associated with hESCs. The deep impact of iPSC technology on the study of cell biology, and especially nuclear reprogramming, was recognized when Prof. Yamanaka, along with Prof. Gurdon, received the Nobel Prize for Physiology or Medicine in 2012.
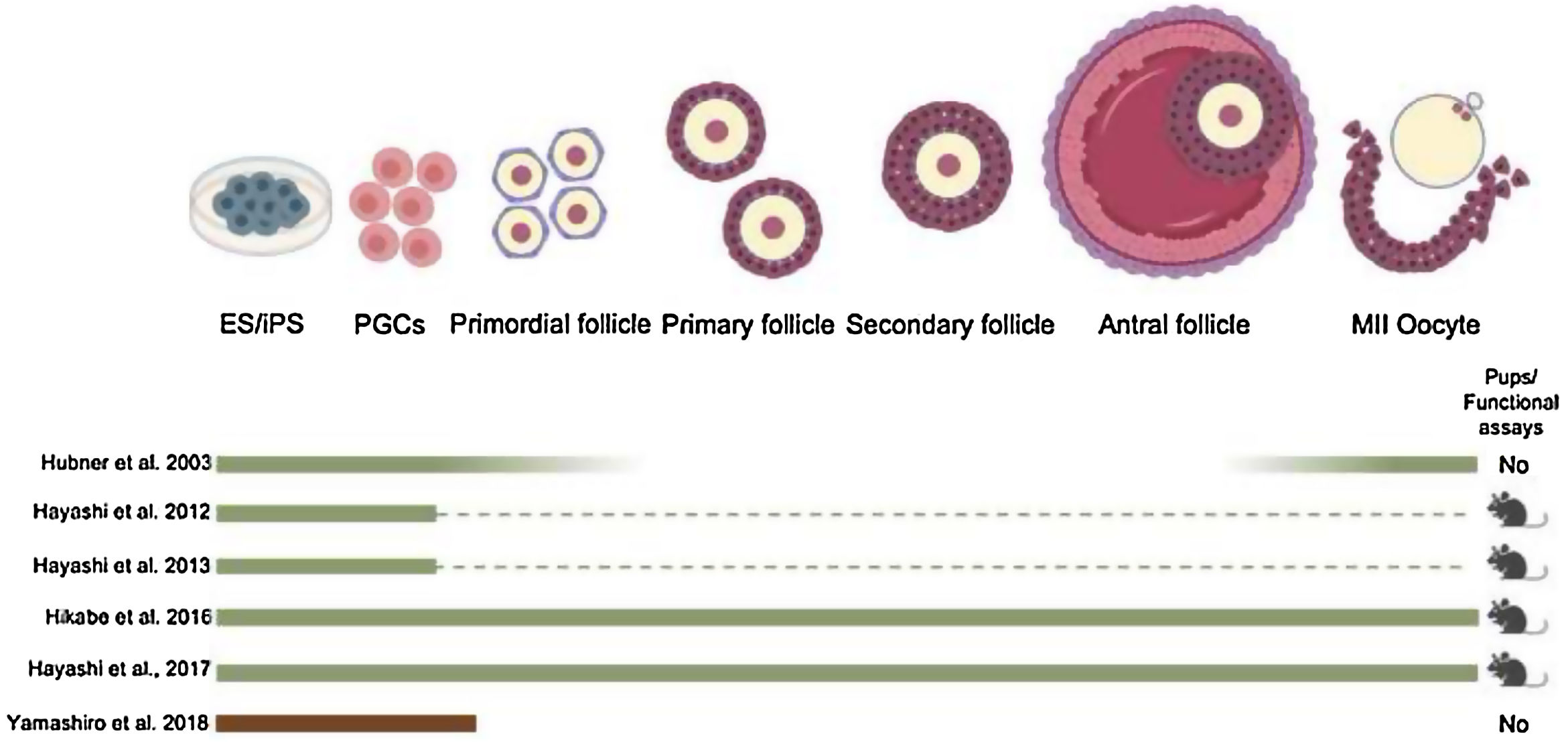
To date, pluripotent stem cells, PSCs (ESC or iPS) have been differentiated to many cell types (mesoderm, ectoderm, endoderm or germ cells), either by directed differentiation or by undirected differentiation. In mammals, development of gametes goes through a fetal stage named primordial germ cells (PGCs) and an important first step to achieve functional gametes from PSCs is to reach the PGC state. Putative PGC-like cells have already been derived in vitro from hESCs for the very first time in human model (Clark et al., 2004) (Fig. 1). As we have just mentioned, the second step for male germ cell differentiation is reaching the post-meiotic stage of spermatozoa. However, still differentiation past the PGC stage has proven challenging in human. The most relevant achievement was the overexpression of specific male germ cell genes, such as deleted in azoospermia (DAZ), deleted in azoospermia like (DAZL) and BOULE (Kee et al., 2009) (Fig. 1) while other groups have attempted supplementing the differentiation medium with growth factors and inhibitors (Eguizabal et al., 2011) (Fig. 1). The same year, in a mouse model, it has been demonstrated that transplanting in vitro produced PGC-like cells from PSCs into mouse testis resulted in functional sperm. That was capable of producing healthy and fertile offspring (Hayashi et al., 2011) (Fig. 1), representing the most important proof of functional male germ cells from mouse PSCs.
Few years later, functional spermatids from mouse PSC were obtained through a fully in vitro protocol (Zhou et al., 2016) (Fig. 1). In 2012, Prof. Hayashi's group used female mPSCs and induced them into PGC-like cells, which were then aggregated with fetal ovarian somatic cells and transplanted under the ovarian bursa. PGC-derived immature oocytes were obtained and were subsequently matured and fertilized in vitro. The resulting embryos were transplanted into foster mothers and contribute to fertile offsprings (Hayashi et al., 2012) (Fig. 1). Later, same Japanese group, reconstituted the complete process of oogenesis in vitro from mESCs and iPSCs to generate functional oocyte-like cells (Hikabe et al., 2016) (Fig. 1). Finally the most recent and unique achievement in human model is the robust derivation of oogonia from human iPS cells but still many obstacles have been to be studied to generate functional oocytes (Yamashiro et al., 2018) (Fig. 1).
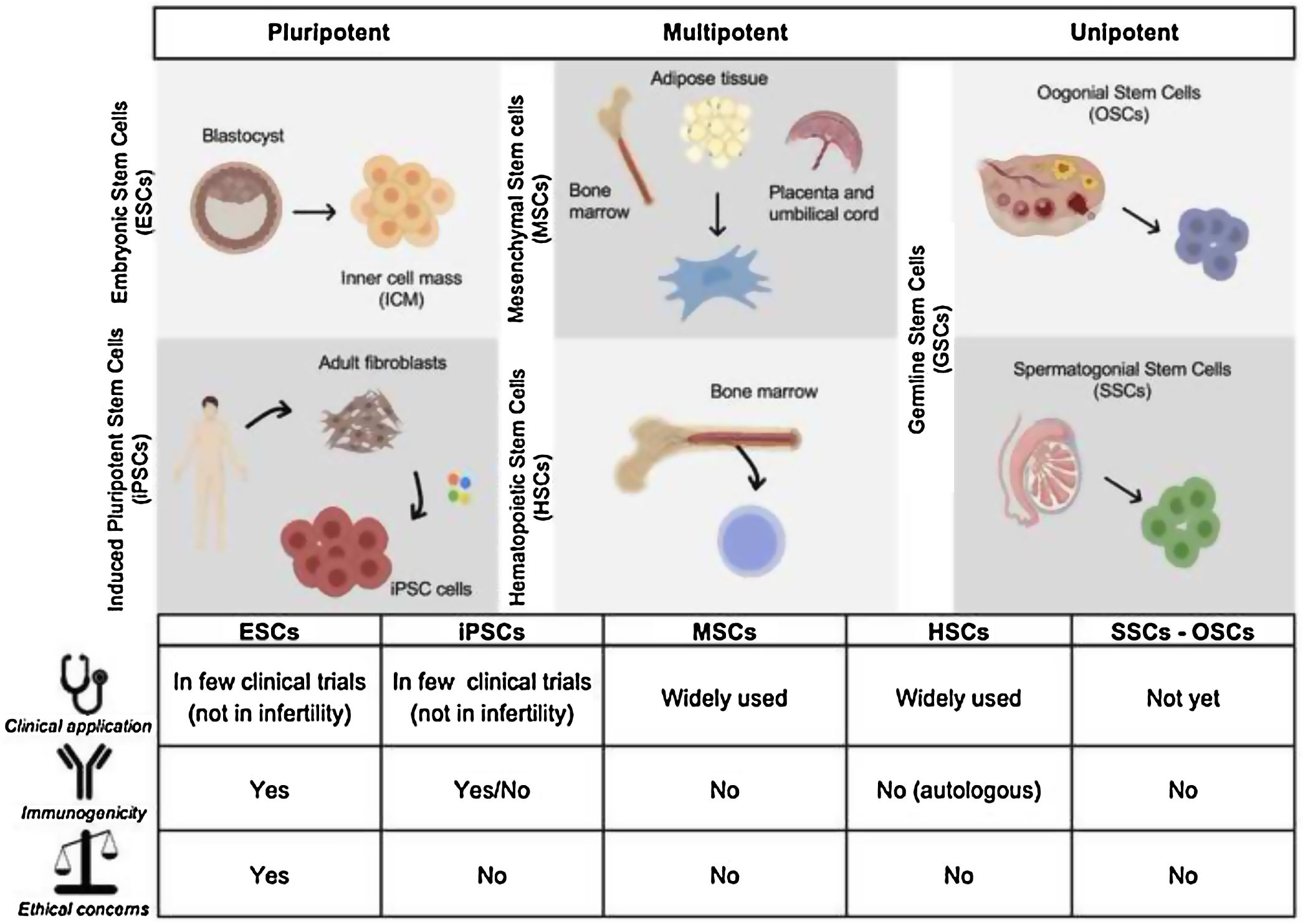
What are stem cells? Potential therapeutic applications in reproductive medicineStem cells are undifferentiated cells that have the ability to extensively proliferate and differentiate into different specialized cells (Eguizabal et al., 2019; Volarevic et al., 2014). There are five main types of stem cells that include embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs) and spermatogonial or oogonial stem cells (SSCs and OSC respectively) (Fig. 2).
Embryonic Stem Cells (ESCs) are pluripotent cells derived from the inner cell mass of the blastocyst (Thomson et al., 1998). ESCs mayor characteristics include self-renewal ability, prolonged proliferation in the pluripotent state and can differentiate into three germ layer cell types (ectodem, mesoderm and endoderm) and also germ cells. Prof. Yamanaka research group generated pluripotent cells by reprograming somatic cells with four transcription factors Oct4, Sox2, Klf4, and c-Myc generating induced Pluripotent Stem Cells (iPS) cell lines (Takahashi et al., 2007; Takahashi and Yamanaka, 2006). These cells have common characteristics with ESCs. Despite sharing same features, the use of ESCs has ethical concern whereas iPSCs do not have this problem. Nowadays there are more than 30 clinical trials that are treating several diseases by using derived cells from human ESCs/iPSC (Eguizabal et al., 2019) but none of them yet to treat infertility related problems. The generation of “artificial” male and female gametes from pluripotent stem cells (ESCs and iPSCs) could be a potential option as an alternative source of gametes or as a model system that recapitulate gametogenesis in vivo.
Mesenchymal Stem Cells (MSCs) are adult multipotent non-hematopoietic stem cells derived from the mesoderm. Possess limited self-renewal and differentiation capability. MSCs can be derived from several adult tissues, such as bone marrow, adipose tissue, placenta, umbilical cord, and peripheral blood (He and von Schwarz, 2021; Volarevic et al., 2014; Zhao et al., 2019). Their main advantage is the efficiency of isolation from other tissues avoiding ethical concerns. Laboratory experiments and clinical trials are now using MSCs for widely disorders applications but also potential application to female infertility disorders (ovarian dysfunction and endometrial disorders) and also in male infertility (erectile disfunction) (He and von Schwarz, 2021; Zhao et al., 2019).
Another mesoderm derived multipotent cells are HSCs, the stem cells that give rise blood cells. This process occurs in the bone marrow, in the core of most bones. The hematopoietic stem and progenitor cell from umbilical cord blood (UCB) and bone marrow (BM) are expressing CD133 and CD34 markers. These HSCs also confer such regenerative properties, specifically BM stem cells selected for CD133 expression, which are considered to be the most immature of the hematopoietic progenitor cells. Clinical trails transfering CD133+ cells promote cell proliferation and neoangiogenesis in endometrium (Santamaria et al., 2016).
SSCs and OSCs are germ line specific stem cells derived from the testicular and ovarian tissue respectively (Volarevic et al., 2014). These are unipotent cells that can derive into mature gametes (sperm and oocytes, respectively). Although these cells would be a great approach to treat infertility the number of SSCs and OSCs in the testis and ovary is relatively small which makes them more difficult to cultivate and expand them in vitro compared to other cell type (ESCs or iPSCs) (Wang et al., 2019). The spermatogenesis is a tightly regulated process that takes place in the seminiferous tubules within the testis. This process starts with SSCs that either self-renew to keep the stem cell pool intact or differentiate to ensure the continuous, life long production of haploid sperm cells. SSC therapy in humans is not clinically applied yet. Nevertheless, cryo-preservation of testicular biopsies is now offered at various centers wordwide. Translational studies on some aspects of the therapy are still required before bringing SSC therapy to the clinic (Vassena et al., 2015) and we will discuss it more in detail later.
OSCs are proposed to be a native population of adult stem cells that could be found in ovaries. They would be unipotent cells capable to proliferate, self-renew and regenerate the pool of oocytes after asymmetric division. However, the theory of the existence of an OSC pool remains contro-versial. Although commercial enterprises are actively studying the possibility of using putative OSCs to treat some kinds of infertility, there is currently no consensus on their existence, origin and functionality (Vassena et al., 2015). Further studies are needed to answer the questions related with the OSCs.
Within the many types of stem cells such as patient-specific induced pluripotent stem cells (iPSCs), embryonic stem cells (ESCs), germline stem cells, whether male spermatogonial stem cells (SSCs) or female oogonial stem cells (OSCs), as well as mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs), most of them may be used as therapeutic tools for infertility treatments (Fig. 2) (Ilic et al., 2019; Zhao et al., 2019). In the next sections, we will cover the potential use of stem cells-derived gametes for treating female and male infertility related diseases in the future.
Pluripotent stem cell differentiation protocols toward artificial gametes in miceIn mammals, development of gametes goes through a fetal stage termed primordial germ cells (PGCs). Mouse PGCs first become identifiable as a cluster of approximately 40 cells at the allantois at around embryonic day 7.25 (∼E7.25) (Chiquoine, 1954; Ginsburg et al., 1990). They migrate to the developing hindgut endoderm at ∼E7.75, into the mesentery at ∼E9.5, and colonize the genital ridges at ∼E10.5 (Molyneaux et al., 2001; Richardson and Lehmann, 2010; Tam and Snow, 1981). A key event that occurs in PGCs during this proliferative phase both in the male and female is epigenetic reprogramming, most notably, a genome-wide DNA demethylation that includes the erasure of genomic imprinting (Saitou and Yamaji, 2012). The X chromosome is also reactivated in female germ cells (Seki et al., 2005). Finally, following reprogramming, PGCs enter a phase of global re-methylation, resulting in a highly methylated sperm or partially methylated oocyte genome (Smith and Meissner, 2013).
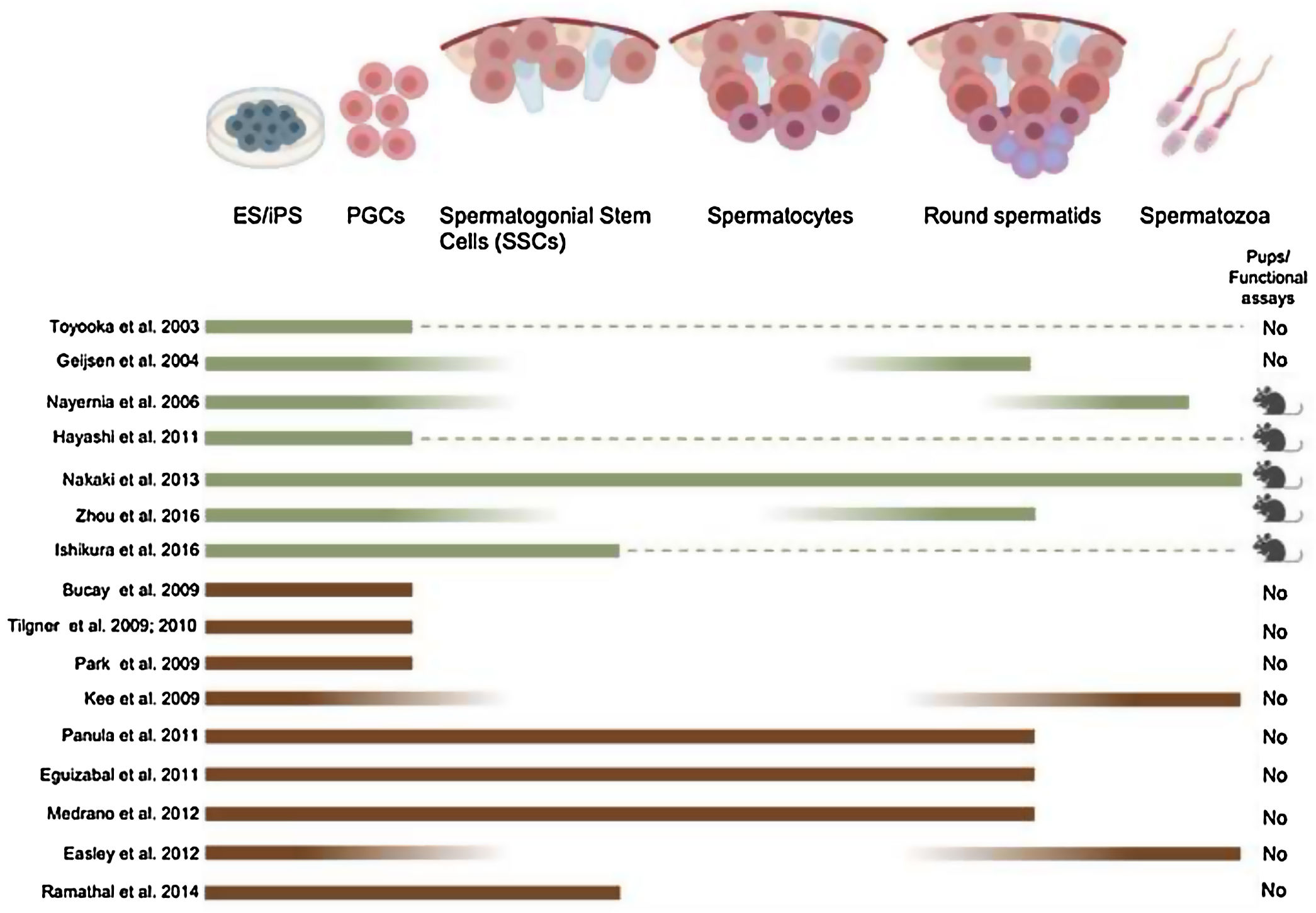
From the last two decades, it has become clear that mouse PSCs can differentiate into PGCs (Fig. 3). Putative PGCs were derived in vitro from mESC (Hübner et al., 2003; Toyooka et al., 2003; Geijsen et al., 2004; Nayernia et al., 2006; Eguizabal et al., 2009; Nakaki et al., 2013; Ishikura et al., 2016; Zhou et al., 2016) and miPSC by using several differentiating culture conditions (growth factors, embryoid body formation, among others). In 2011 and 2012, mouse PGCs were generated in vitro using a novel approach (Hayashi et al., 2012, 2011). It was demonstrated that the majority of the EpiSC have lost their competency toward a PGC fate (Hayashi and Surani, 2009). To circumvent this hurdle, naïve mESCs derived from the preimplantation epiblast were differentiated toward an intermediate epiblast-like stage in the presence of activin A, resembling more the early pluripotent epiblast cells that give rise to PGCs in vivo (Hayashi et al., 2012, 2011). The PGCs formed using this novel approach could eventually lead to functional oocytes and sperm capable of generating new offspring.
Obtaining PGCs from pluripotent cells is the first step in the differentiation toward post-meiotic spermatozoa. However, further differentiation has proven challenging in vitro. The strategies employed to induce it include supplementing the differentiation medium with the growth factors such as bone morphogenetic proteins (Geijsen et al., 2004), N2B27, Activin A, Fibroblast growth factor 2 (Hayashi et al., 2011) and retinoid acid (Cai et al., 2013; Eguizabal et al., 2009; Nayernia et al., 2006) (Fig. 3). Moreover, the overexpression of specific male germ cell genes such as Blimp1, PRDM14 and TFAP2C (Nakaki et al., 2013) have been used to guide differentiation toward spermatozoa (Fig. 3). A relevant step is also to recreate in vitro the testicular niche for a better differentiation of pluripotent cells, where male pre-meiotic germ cells can be differentiated along with Sertoli and Leydig cells (Bucay et al., 2009; Eguizabal et al., 2011) (Fig. 3).
As mentioned before, correct epigenetic reprogramming is essential for the development of functional gametes in general. However, although the male germ cells obtained in vitro follow a normal methylation pattern for some imprinted genes (H19, IGF2, SNRPN), the methylation pattern and offspring were abnormal (Nayernia et al., 2006) in this study, when using a totally in vitro protocol for the generation of mouse male germ cells (Fig. 3). Healthy offspring with normal methylation patterns of imprinted genes are conversely obtained when gametogenesis is resumed in in vivo conditions (Hayashi et al., 2011; Nakaki et al., 2013) (Fig. 3). An important proof of functionality of male germ cells derived from pluripotent cells is to transplant them into the testis of sterile individuals, or performing ICSI, as demonstrated in the mouse (Geijsen et al., 2004; Hayashi et al., 2011; Nakaki et al., 2013) (Fig. 3). It has been recently shown that transplanting in vitro produced PGC-like cells into the mouse testis could be used to obtain functional mouse sperm, capable of producing healthy and fertile offspring (Hayashi et al., 2011). From the recent scientific literature, a Chinese group developed a fully in vitro protocol to generate mouse spermatids from mouse PSCs (Zhou et al., 2016) (Fig. 3).
From the female side, authors demonstrated that mESCs are able to form follicle-like structures with oocyte-like cells, but which were not able to progress into meiosis (Hübner et al., 2003) (Fig. 4). Research conducted using mouse pluripotent stem cells (mPSCs) to direct differentiation into the female germ line has so lagged far behind. As mentioned above, this outcome was achieved in the mouse model by Prof. Hayashi's team in 2012 making use of PGCs obtained from female EpiLCs, which were subsequently aggregated with somatic cells from embryonic ovaries and transplanted under the ovarian bursa (Hayashi et al., 2012) (Fig. 4). PGC-derived immature oocytes were obtained and were subsequently matured and fertilized in vitro, and embryos were transplanted into foster mothers. While this in vitro approach was less efficient than the use of in vivo derived PGCs, healthy and fertile offspring were obtained. Still, PGC themselves were often not completely ‘normal’ as, for example, the second-generation PGCs often produced fragile and misshapen oocytes, sometimes lacking supporting granulosa cells. After fertilization of these artificial oocytes, abnormal pronucleus formation was often observed, which could be responsible for the lower rates of postimplantation development compared with controls. In order to improve this protocol, few years later, the same Japanese group achieved fuctional oocytes from mPSC in a fully in vitro differentiating protocol (Hikabe et al., 2016) (Fig. 4). They obtained better results but still some important issues must be improved. For example, the difference in efficiency creating pups depending on the source of pluripotent stem cells used (3.5% from mES cells vs. 0.9% from miPS cells). The maturation step is critical in oogenesis in vitro, the in vitro oocytes are having low mitochondria copy number and still are frequent aneuploidy embryos in comparison with in vivo oocytes. Furthermore, the extracellular matrix plays an important role in the dormant state of primordial follicles, which can also be produced in culture. In conclusion, whereas it is possible to generate in vitro functional oocytes from mouse PSC still there is room to improve efficiency, maturity and ploidy in the in vitro PSC-derived oocytes.
Pluripotent stem cell differentiation protocols toward artificial gametes in humansIn humans, PGCs can be first identified at the end of the third week of gestation in the wall of the yolk sac. After migration, human PGCs colonize the gonadal tissue at 5–6 weeks of gestation. In order to restore the developmental potential in the next life cycle, PGCs undergo global epigenetic reprogramming through chromatin remodeling, erasure of genomic imprints and extensive DNA demethylation (Eguizabal et al., 2016; Hajkova et al., 2008). The X chromosome is also reactivated in female germ cells (Seki et al., 2005). Finally, following reprogramming, PGCs enter a phase of global re-methylation, resulting in a highly methylated sperm or partially methylated oocyte genome (Smith and Meissner, 2013). In the last few years it has become clear that PSCs in general can differentiate into PGCs. In the human species several authors could described an increase of later stage PGC markers differentiating human PSC (Clark et al., 2004) by using embryoid body assay (Bucay et al., 2009), monolayers (Tilgner et al., 2010, 2008) or co-culture with fetal gonads (Park et al., 2009) (Fig. 3).
Obtaining PGCs from pluripotent cells is the first step in the differentiation toward post-meiotic spermatozoa. However, further differentiation toward post-meiotic germ cells has proven challenging in vitro up to date. The methods used to favor it include supplementing the differentiation medium with growth factors such as BMP4 (Tilgner et al., 2008), retinoid acid (Eguizabal et al., 2011), inhibitors (R115866) (Eguizabal et al., 2011), and hormones such as Insulin and Testosterone (Easley et al., 2012) (Fig. 4). Moreover, the overexpression of specific male germ cell genes such as deleted in azoospermia (DAZ), deleted in azoospermia like (DAZL), BOULE (Kee et al., 2009; Medrano et al., 2012; Panula et al., 2011) and VASA (Medrano et al., 2012; Tilgner et al., 2010, 2008), have been used to guide differentiation toward spermatozoa (Fig. 4). A relevant step is also to recreate in vitro the testicular niche for a better differentiation of pluripotent cells, where male pre-meiotic germ cells can be differentiated along with Sertoli and Leydig cells (Bucay et al., 2009; Easley et al., 2012; Eguizabal et al., 2011) (Fig. 4).
As described before, correct epigenetic reprogramming is essential for the development of functional gametes in general. Some groups reported the obtention of in vitrohuman male germ cells with a normal methylation pattern for an imprinted gene (H19) (Eguizabal et al., 2011; Kee et al., 2009; Medrano et al., 2012; Panula et al., 2011; Park et al., 2009; Tilgner et al., 2008). An important proof of functionality of male germ cells from pluripotent cells is to transplant them into the testis of sterile individuals as demonstrated in the mouse, however, in humans is not possible. There is only one single study reported in 2014, that generated human iPSCs (hiPSC) from azoospermic and normospermic males that were transplanted into sterile mouse testis that were able to partially colonize the testicular niche and showing signs of early stage spermatogenesis (Ramathal et al., 2014), raising the question of whether an initial in vitro differentiation step is needed at all. From the recent scientific literature, it seems that so far there is still the need of a natural testicular niche in order to obtain mature functional spermatozoa, and that in vitro production of functional spermatozoa is currently not possible in human model (Fig. 4).
Recently, a Japanese group for the very first time, has induced primordial germ cell-like cells (hPGCLCs) from human iPS cells and further differentiation into oogonia (Yamashiro et al., 2018) mimicking the fully in vitro protocol from Prof. Hayashi published in 2016 (Hikabe et al., 2016) (Fig. 4). However, further differentiation to mature and functional oocytes has not been achieved due to the lack of a normal X chromosome reactivation and epigenetic reprogramming together with the lack of granulosa cells during the in vitro differentiation process. Further studies are needed to get functional female gametes from human pluripotent stem cells for future applications.
Current stem-cell therapies for male infertility related disordersSSC-based strategies for male infertility are an alternative in cases where it is not possible to cryopreserve semen. For that, well characterization and proliferation of the SSCs are being studied (Gauthier-Fisher et al., 2020). Cancer is a major cause of death in children and adolescents. However, as a result of notable improvements in treatments, cancer death rates in childhood and adolescence have declined significantly. The results of the European and American data suggest that a survival of 80% of children and adolescents diagnosed with cancer can be expected. Unfortunately, the treatments used to cure cancer, such as chemotherapy and radiotherapy, due to their exposure, dose and their gonadotoxic effect, can damage the spermatogenic stem cells (SSCs) in the testicles of these patients, causing infertility problems to long-term, in most cases permanent. In addition to these malignant diseases, there are other types of genetic syndromes, such as Klinefelter Syndrome that can lead to the premature loss of germline stem cells in children.
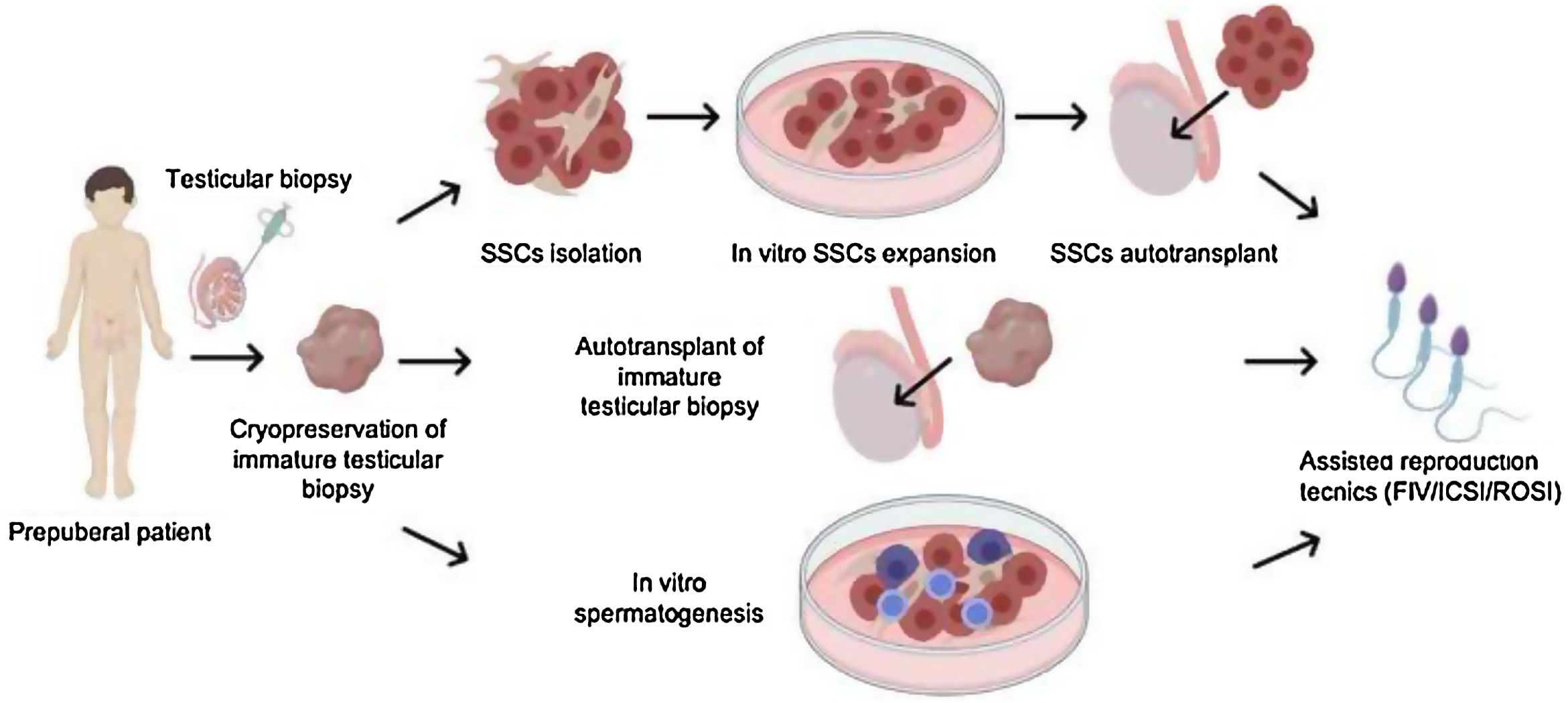
Different strategies have been developed to safeguard the fertility of these young patients, first of all cryopreservation of sperm, as a first-line fertility preservation treatment, in the case of adolescent patients, performed routinely. However, for some adolescents and clearly for prepubertal males this option is not possible. For both, the cryopreservation of testicular tissue is the only experimental alternative that exists to preserve their fertility (Goossens et al., 2020).
As mentioned above, once the testicular biopsy has been cryopreserved and the patient has fertility problems in the future, then the moment arises to restore their fertility.
Currently there are three strategies: (1) in vitro expansion and autotransplantation of SSCs; (2) autotransplantation of testicular biopsy and (3) in vitro spermatogenesis as both three are indicated in Fig. 5. Among the different experimental fertility restoration strategies described, there are still problems that must be solved thanks to basic research work together with the clinic in the coming years. Fertility programs in children and adolescents are increasingly known. Since 2016 we have started a Fertility preservation program in Osakidetza at Basque Center for Blood Transfusion and Human Tissues focused on prepubertal children with infertility related disorders such as cancer or Klinefelter syndrome, among other pathologies. However, there are still hospitals that do not know it, and therefore it is necessary to disseminate well and create guidelines or recommendations so that these patients can benefit from these experimental programs in the future.
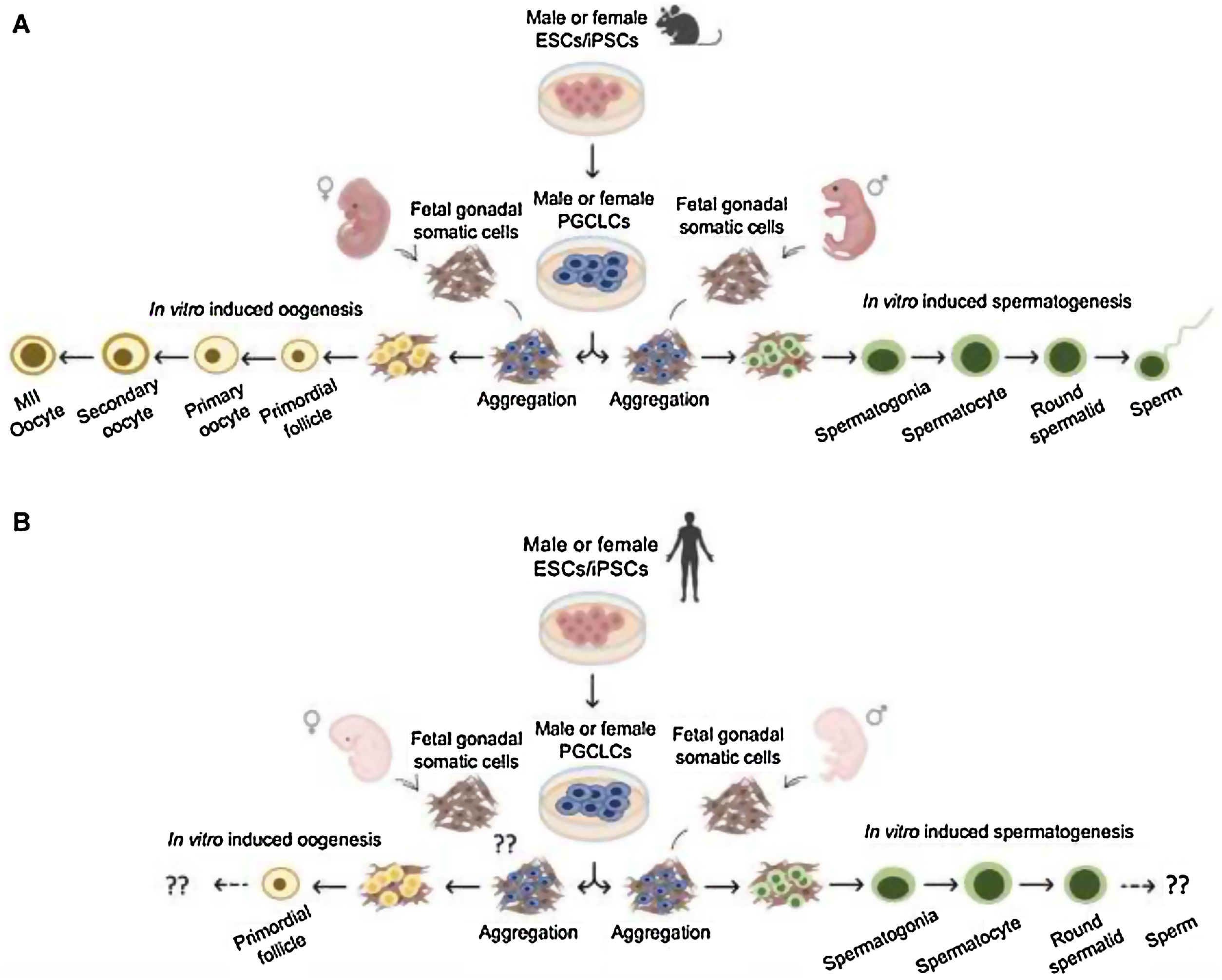
Conclusions and future perspectivesIn vitro gametogenesis using PSCs (ESCs or iPSCs) can provide functional gametes in mice (Fig. 6A). These technologies can be beneficial in two different ways, either as an alternative source of gametes or as a model system that recapitulate gametogenesis in vivo. The quality of the gametes produced in vitro is the critical outcome. Recent experiments in mice have clearly shown that the quality of the gametes in vitro is inferior to that of the gametes in vivo, due, at least in part, to aberrant differentiation processes under suboptimal culture conditions. Further refinement of the culture conditions can thus be expected to increase the utility of in vitro gametogenesis. Reproductive biology and in vitro gametogenesis are most advanced in mice, and it will not be easy to generalize the murine gametogenesis method to humans and other animals. In particular, germ cell development requires the shortest length of time in mice. Because of this problem, together with the above-mentioned issues with quality, the study of in vitro gametogenesis has only just begun and constant efforts will be required for development of the technology.
Besides many promising experimental stem cell-based approaches are under development to restore male fertility in prepuberal boys. Recently, important milestones have been achieved in this field, SSC transplantation has been translated to human cadaver testis, proof-of-concept has been obtained for testicular tissue grafting in non-human primates, and important steps have been taken in establishing spermatogenesis in vitro. However, until successful clinical trials demonstrating safety and efficacy of fertility restoration have been conducted, testicular tissue cryopreservation for fertility preservation should remain experimental.
Finally, although in vitro germ cell differentiation from human PSCs is often mentioned as a potential future option for infertility treatment, has to take in mind that the actual protocols do not allow sufficient differentiation of fully matured sperm or oocytes, and even if possible, testing the functionality of them by fertilizing oocytes and producing offspring will present many ethical concerns, which need to be addressed very carefully (Fig. 6B). However, iPSCs modeling strategies to study the fundamental aspects of germ cell specification can be considered as crucial steps to gain more knowledge on germ cell development as well as differentiation processes. In conclusion, the generation of artifical gametes from human iPS is still a “distant prospect”.
Authors’ contributionsM.M-I. and C.E. conceived and designed the study and performed the literature search and manuscript writing. All authors revised and approved the final manuscript.
FundingThis work was funded by the Health Department of the Basque Government (Grant 2019111068, 2019/4703 and 2020333032) and Jesus de Gangoiti Barrera Foundation.
Conflicts of interestThe authors indicate no potential conflicts of interest.
We apologize to our colleagues whose work was not cited due to space limitations.