STAT-ON™ is an objective tool that registers ON-OFF fluctuations making possible to know the state of the patient at every moment of the day in normal life. Our aim was to analyze the opinion of different Parkinson's disease experts about the STAT-ON™ tool after using the device in a real clinical practice setting (RCPS).
MethodsSTAT-ON™ was provided by the Company Sense4Care to Spanish neurologists for using it in a RCPS. Each neurologist had the device for at least three months and could use it in PD patients at his/her own discretion. In February 2020, a survey with 30 questions was sent to all participants.
ResultsTwo thirds of neurologists (53.8% females; mean age 44.9±9 years old) worked in a Movement Disorders Unit, the average experience in PD was 16±6.9 years, and 40.7% of them had previously used other devices. A total of 119 evaluations were performed in 114 patients (range 2–9 by neurologist; mean 4.5±2.3). STAT-ON™ was considered “quite” to “very useful” by 74% of the neurologists with an overall opinion of 6.9±1.7 (0, worst; 10, best). STAT-ON™ was considered better than diaries by 70.3% of neurologists and a useful tool for the identification of patients with advanced PD by 81.5%. Proper identification of freezing of gait episodes and falls were frequent limitations reported.
ConclusionSTAT-ON™ could be a useful device for using in PD patients in clinical practice.
STAT-ON es un dispositivo que registra las fluctuaciones on-off que permite conocer el estado del paciente con enfermedad de Parkinson (EP) en cada momento del día.
Nuestro objetivo fue analizar la opinión de diferentes expertos en EP sobre STAT-ON, después de usar el dispositivo en un entorno de práctica clínica real (PCR).
MétodosSTAT-ON fue proporcionado por la compañía Sense4Care a neurólogos españoles para usarlo en PCR. Cada neurólogo dispuso del dispositivo durante al menos tres meses y podía usarlo en pacientes con EP, según su criterio. En febrero de 2020, se envió una encuesta con 30 preguntas a todos los participantes.
ResultadosDos tercios de los neurólogos (53,8% mujeres; edad promedio 44,9 ± 9 años) trabajaban en una Unidad de Trastornos del Movimiento, con una experiencia en EP de 16 ± 6,9 años, habiendo el 40,7% usado otros dispositivos previamente. Se realizaron un total de 119 evaluaciones en 114 pacientes (rango dos a nueve por neurólogo; media 4,5 ± 2,3). STAT-ON fue considerado «bastante» a «muy útil» por el 74% de los neurólogos, con una opinión general de 6,9 ± 1,7 (0, peor; 10, mejor). STAT-ON fue considerado mejor que los diarios por el 70,3% de los neurólogos y una herramienta útil para la identificación de pacientes con EP avanzada por un 81,5%. La identificación adecuada de los episodios de congelación de la marcha y las caídas fueron las limitaciones más reportadas.
ConclusionesSTAT-ON podría ser un dispositivo útil para usar en la PCR.
The symptomatic treatment of Parkinson's disease (PD) with current therapies is effective. However, over time, motor complications appear, impacting on the patient's quality of life and the autonomy for performing daily-living activities. Although previous reports demonstrated that approximately 40% of PD patients who were treated with levodopa for 4–6 years experienced motor complications,1 studies, examining more specifically wearing-off, have observed that this is a potentially early phenomenon. As an example, this manifestation affects already 42% of the PD patients with <2.5 years disease when a screening tool was used (WOQ-19) and 21% according to a neurologist assessment.2 Gathering accurate information about the clinical status of the patient is essential for planning treatment and assessing its effect. Daily motor function assessment with paper diaries for detecting time periods of the day during the OFF state, ON state without dyskinesia and ON state with disabling or non-disabling dyskinesia, result in issues of compliance and reliability.3 Electronic diaries have the advantages of facilitating the transmission of the information directly by PD patients. Still both type of diaries, paper and electronic, are limited by compliance and reliability issues. Importantly, previous studies have shown that diaries resulted in average response compliance of approximately 1h.4 Therefore, more objective and precise systems are necessary to complement diaries. Wearable inertial sensors have been widely used to monitor the motor status and analyze symptoms of PD such as tremor, bradykinesia, or dyskinesia.5–12
The REMPARK system (REMPARK Personal health device for the remote and autonomous management of Parkinson's disease, FP7 project REMPARK ICT-287677) is a wearable system, developed between 2011 and 2015, to monitor motor states through a system requiring a single sensor.13,14 Analogous to the “Holter” for the monitoring of cardiac parameters, the REMPARK system has been designed to record the patient's motor condition during daylight hours and to aid in managing the disease via the smartphone. By using a single sensor (an inertial measurement unit called 9×3) advanced algorithms, and signal processing methods, this system monitors and records PD motor manifestations.15 The device contains an accelerometer, rechargeable battery for 7 days, data storage of at least 1 year. It is able to acquire and store raw inertial data for artificial intelligence algorithmic training purposes. It has been marketed as STAT-ON™ by the Company Sense4Care. STAT-ON™ provides information about bradykinesia index based on fluidity movements, dyskinesia, freezing of gait (FOG), ON and OFF states, gait parameters (speed of stride, cadence, time walking, step length, number of steps), number of falls and energy expenditure and postures. STAT-ON™ demonstrated an overall classification accuracy of 92.2% to detect OFF periods under real conditions of use in 23 advanced PD patients wearing the kinematic sensor for 1–3 days at home.16 More recently, it demonstrated in 41 patients with moderate to severe idiopathic PD using the system in the sensitivity and 88% specificity in detecting OFF states.16,17 Although there are no cost/effectiveness studies, the use of the device could, hypothetically, help to identify motor fluctuations properly, which could improve with an adequate treatment optimization. This could help reduce costs (e.g., emergency assistance, institutionalization, etc.).
Despite the multiple studies showing the accuracy of the system to detect motor fluctuations, there is no information about its possible clinical utility. Here, we report the opinion of different PD expert neurologists about the STAT-ON™ utility in a real clinical practice setting (RCPS).
MethodsCE Mark Medical Device STAT-ON™ was provided by the Company Sense4Care to different Spanish neurologists for using it in a RCPS between October and December 2019. All neurologists were selected because they were PD experts. Each neurologist had the opportunity to use the device in PD patients for at least 3 months at his/her own discretion. In February 2020, a survey with 30 questions on STAT-ON™ utility was sent to all participants (Supplementary Material). The data was anonymized and three waves were made to obtain the responses. The participation was voluntary.
Standard protocol approvals, registrations, and patient consentsSince the present study was an anonymized opinion survey about the usefulness of a CE marketed device for clinical practice use, approval was not sought from any ethics committee. No patient data was recorded.
Data availabilityThe protocol, statistical analysis plan and unidentifiable data are available upon request.
Data analysisData were processed using SPSS 20.0 for Windows. A first descriptive analysis was carried out in which the results were shown in counts (percentages) or as mean±deviation according to the type of variable. Depending on the results of the first analysis, correlation between specific variables were analyzed. The Student's t-test, Mann–Whitney U test, Chi-square test or Fisher test were used as appropriate (normality assumption was verified with a one-sample Kolmogorov–Smirnov test). Spearman's or Pearson's correlation coefficient were also used for analyzing the relationship between continuous variables depending also on the data distribution. Correlations were considered weak for coefficient values ≤0.29, moderate for values between 0.30 and 0.59, and strong for values ≥0.60. The value of p was considered significant when it was <0.05.
ResultsA total of 37 neurologists from 35 Spanish centers received the device from the manufacturer for using it in PD patients in a RCPS. Twenty-seven surveys (73%) were received (53.8% females; mean age 44.9±9 years old).
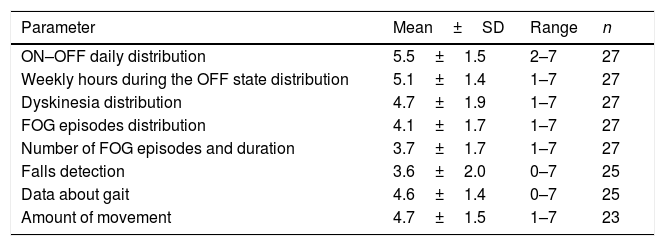
Two thirds of the neurologists worked in a Movement Disorders Unit, the mean experience on PD was of 16±6.9 years, and 40.7% of them had used previously different devices for PD. A total of 119 evaluations in 114 patients (range from 2 to 9 per neurologist; mean 4.5±2.3) were performed. Using a score from 1 (unhelpful) to 7 (very helpful), the opinion about different usability aspects was recorded (Table 1). ON-OFF daily distribution was the best scored (5.5±1.5) whereas falls detection the worst (3.6±2). The subjective general opinion about the device (from 0, the worst, to 10, the best) was 6.9±1.7.
Score from 1 (unhelpful) to 7 (very helpful) of the opinion about different usability aspects of the STAT-ON device.
| Parameter | Mean±SD | Range | n |
|---|---|---|---|
| ON–OFF daily distribution | 5.5±1.5 | 2–7 | 27 |
| Weekly hours during the OFF state distribution | 5.1±1.4 | 1–7 | 27 |
| Dyskinesia distribution | 4.7±1.9 | 1–7 | 27 |
| FOG episodes distribution | 4.1±1.7 | 1–7 | 27 |
| Number of FOG episodes and duration | 3.7±1.7 | 1–7 | 27 |
| Falls detection | 3.6±2.0 | 0–7 | 25 |
| Data about gait | 4.6±1.4 | 0–7 | 25 |
| Amount of movement | 4.7±1.5 | 1–7 | 23 |
FOG, freezing of gait; SD, standard deviation.
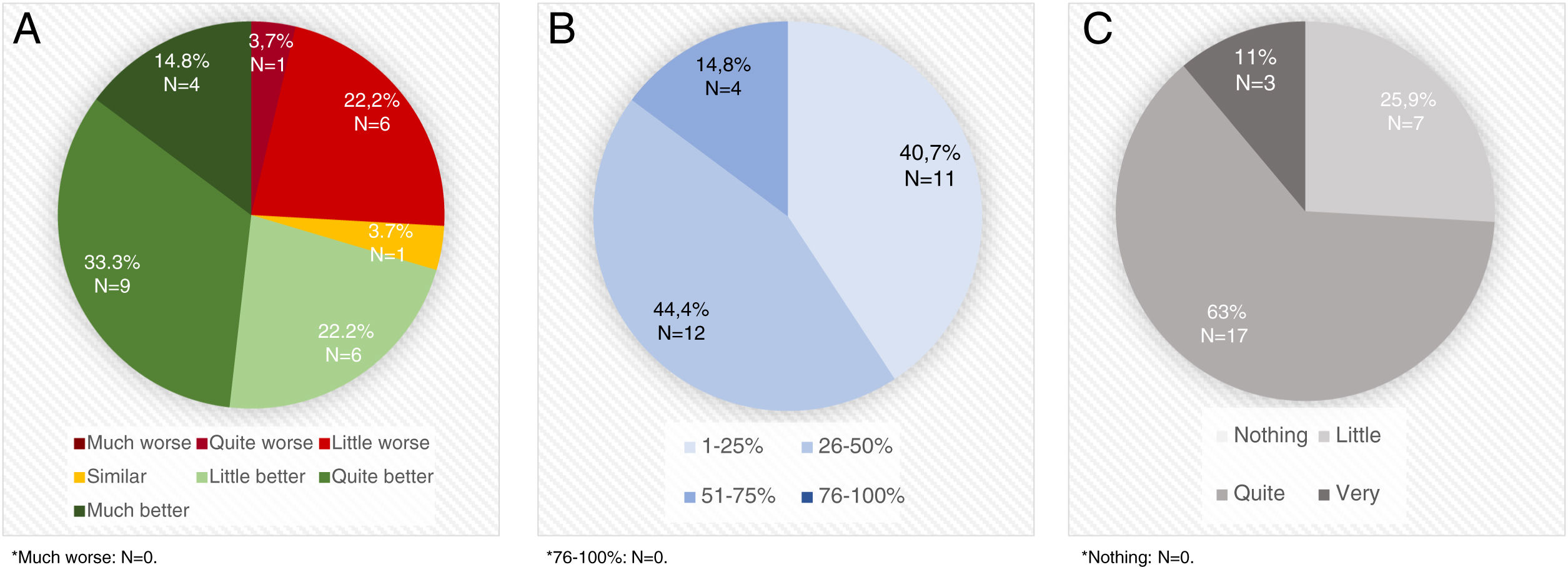
STAT-ON™ was considered better than the diaries by 70.3% of the neurologists (Fig. 1A) and a useful device for identifying advanced PD patients by 81.5%. A high percentage of PD patients were considered that could benefit from using STAT-ON™ (Fig. 1B). STAT-ON™ was considered from quite to very useful by 74% of the neurologists (Fig. 1C). A significant moderate positive correlation was observed between the number of assessments performed and the general opinion about the device (r=0.403; p=0.046). By the contrary, no differences were observed in the general opinion about the device (from 0 to 10) with respect to the age of the PD expert (r=0.030; p=0.830), the number of years treating PD patients (r=0.032; p=0.881), the previous use of devices for PD (7.2±2.1 vs 6.9±1.5; p=0.703), and the practice setting (Movement Disorders Unit vs. standard outpatient clinic) (7.2±2.3 vs 6.9±1.5; p=0.784).
A. Opinion about STAT-ONTM compared to OFF-ON diaries. B. Percentage of Parkinson's disease patients (1-25% vs 26-50% vs 51-75% vs76-100% of all Parkinson's disease patients assessed in real clinical practice setting) who, evaluated in daily clinical practice, at the discretion of the evaluator, could benefit from the usage of STAT-ONTM. C. Usefulness of the information collected with the device.
STAT-ON™ was specifically used by 13 of the 27 neurologists (48.1%) in 21 advanced PD patients who were treated with an advanced therapy: 10 deep brain stimulation (DBS); 6 subcutaneous apomorphine infusion; 3 intraduodenal levodopa infusion (2 without information). Only one neurologist considered STAT-ON™ as not useful in these patients. Sixty three percent considered it from quite to very useful (N=11; 2 without answer) for using in this population and with a periodicity that should be individualized (67%).
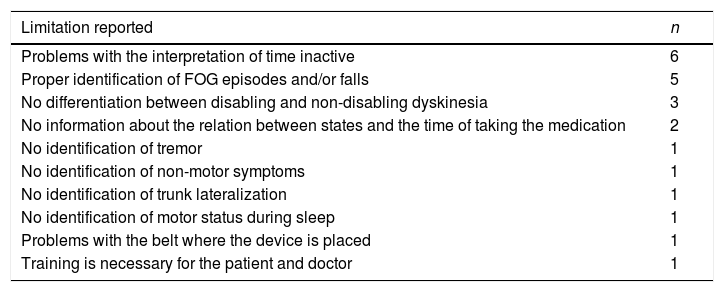
With regards to the limitations of the information provided by the device or other related usability problems, 17 neurologists (63%) demanded other relevant information or found problems (Table 2). The most frequent were problems with the interpretation of time in gray color and proper identification of freezing of gait (FOG) episodes and/or falls.
Limitations of the information provided by the device recorded by the neurologists.
| Limitation reported | n |
|---|---|
| Problems with the interpretation of time inactive | 6 |
| Proper identification of FOG episodes and/or falls | 5 |
| No differentiation between disabling and non-disabling dyskinesia | 3 |
| No information about the relation between states and the time of taking the medication | 2 |
| No identification of tremor | 1 |
| No identification of non-motor symptoms | 1 |
| No identification of trunk lateralization | 1 |
| No identification of motor status during sleep | 1 |
| Problems with the belt where the device is placed | 1 |
| Training is necessary for the patient and doctor | 1 |
FOG, freezing of gait.
Almost 89% of the neurologists considered that the sensor had a pleasant appearance for the patient and that the waist was a proper place for wearing it. Moreover, 85% of the neurologists considered, based on their experience using the system, that the patient's opinion about the device was good. However, the time invested in explaining the patient the device and the analysis of the results was considered a limitation by the 40.7% of the neurologists. Importantly, 89% would use the device in their daily clinical practice.
In Supplementary Material all the information about the answers to the 30 questions of the survey is shown.
DiscussionThis is the first publication about the opinion of a group of PD experts on the STAT-ON™ system after using it in a RCPS. In general, the opinion was favorable although there are aspects that could be improved. Despite the analysis being based on survey data, it is valuable as it has been carried out by an independent large group of experts in PD, with previous experience with other devices to monitor the disease, and after having jointly evaluated more than 100 patients with this novel system.
STAT-ON™ is a non-invasive Class II A medical device according to Directive 93/42/EEC that has been developed after different R&D European projects since 2009 and being validated in different clinical studies.13–23 However, until now, no information about the opinion of the neurologists on STAT-ON™ after using it in a RCPS had been reported.
In our study, the survey response rate was acceptable (73%).24 Additional strengths of the study were the referred experience on PD of the neurologists, the number of neurologists who participated and the fact that the questionnaire was anonymous and the responses were independent. The best scored parameters about the usability of STAT-ON™ were “ON-OFF daily distribution” and “Weekly hours during the OFF state distribution”, agreeing with the prior evidence on the ability of the system to detect ON-OFF states in PD patients in real conditions.16,17 One of the main advantages of the system is that patients need to wear only a single sensor, making it compatible with an active life. Participants in previous studies considered STAT-ON™ to be user-friendly and were satisfied with the system,16,25 being these findings in accordance with the opinion expressed by the neurologists in the survey.
One of the important findings is that a large majority of the neurologists considered STAT-ON™ to be a better method for monitoring PD patients than diaries. Although diaries have been used in trials as the gold standard, what method is better has not been reported and a trial for comparing PD holter vs PD diary is on-going (https://clinicaltrials.gov/ct2/show/NCT04176302). A second important aspect is, as the result of the survey suggest, the possibility of using STAT-ON™ in a high percentage of PD patients due to the fact that motor fluctuations are frequent and disabling even in early PD patients.26 Finally, a very important finding is that STAT-ON™ could be a useful method for monitoring PD patients under DBS or an infusion therapy.27 Although the experience was limited to 21 patients treated by 13 neurologists, 10 out of 11 that replied to the survey stated that STAT-ON™ could be useful, being this observation interesting because it would allow to monitor the response before and after the therapy. Since these are expensive treatments and not free of complications, this is a great area of interest for using these new systems.
STAT-ON™ is an objective method for monitoring OFF-ON fluctuations. However, the subjective component of the patient's opinion is necessary to know what happens when the patient is not moving as the sensor does not collect data in inactivity periods. This limitation was the most frequently reported and should be taken into account when using STAT-ON™ in patients who spend a lot of time sitting or inactive. Although previous trials demonstrated a high sensitivity and specificity for detecting dyskinesia and FOG,18,20–22 a limitation reported by the neurologists was that the device was not capable of distinguishing disabling and non-disabling dyskinesias. Moreover, the proper identification of FOG episodes and/or falls was the second most frequent limitation reported. Some experts considered that the system overestimated falls in some instances. Integrated information on medication intake hours was also demanded by the experts. Finally, although only one neurologist commented the fact of not informing about non-motor symptoms as a limitation of the system, this is an essential point because non-motor symptoms are frequent and related to motor fluctuations.26,28 Alternatively, the patient could be trained to press the alarm button when a specific non-motor symptoms, such as pain or anxiety, appear and analyze their appearance in relation to the motor state at that moment.29
The device was used a total of 119 times in 114 patients. However, the average use by neurologist was less than five with a range from 2 to 9 times, the device was used for a brief time, and for the vast majority it was their first experience with the system (data not collected) being necessary to learn about the usage of the device, so our findings on the possible usefulness of STAT-ON™ to monitor PD patients in a RCPS should be considered with caution. Although 81.5% of the evaluators considered that the device could be useful for detecting patients with advanced PD, obviously, it is an opinion survey and not a study designed to determine the sensitivity and specificity of the device for detecting advanced PD based on defined criteria (e.g., CDEPA30). The fact that there was a better opinion on the device in more frequent users should be confirmed in future studies to rule out different perception according to technology adoption and exposure. Finally, other biases related to the methodology of the present study cannot be excluded (e.g., question types).
In summary, this is the first opinion survey of a group of PD experts who evaluated the utility of STAT-ON™ in a RCPS. STAT-ON™ could be a useful device for monitoring PD patients in clinical practice. More experience is needed for having a contrasted and confident opinion.
DeclarationsEthics approval and consent to participateThe present study consists of an opinion poll and there are no identifiable data collected from patients and/or participants. Therefore, it does not require the approval of an ethical committee. The first author, Diego Santos García, is a member of an autonomous ethics committee, the “Comité Autonómico de Ética de Investigación de Galicia”, and is fully familiar with the regulations as they apply.
Consent for publicationNot applicable.
Availability of data and materialThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributionsSantos García D.: conception, organization, and execution of the project; statistical analysis; writing of the first draft of the manuscript; evaluation of participants; survey response.
López Ariztegui N.: review and critique; evaluation of participants; survey response.
Cubo E.: review and critique; evaluation of participants; survey response.
Vinagre Aragón A.: review and critique; evaluation of participants; survey response.
García-Ramos R.: review and critique; evaluation of participants; survey response.
Borrué C.: review and critique; evaluation of participants; survey response.
Fernández-Pajarín G.: review and critique; evaluation of participants; survey response.
Caballol N.: review and critique; evaluation of participants; survey response.
Cabo I.: review and critique; evaluation of participants; survey response.
Barrios López J M.: review and critique; evaluation of participants; survey response.
Hernández Vara J.: review and critique; evaluation of participants; survey response.
Ávila Rivera M. A.: review and critique; evaluation of participants; survey response.
Gasca-Salas C.: review and critique; evaluation of participants; survey response.
Escalante S.: review and critique; evaluation of participants; survey response.
Manrique de Lara P.: review and critique; evaluation of participants; survey response.
Pérez Noguera R.: review and critique; evaluation of participants; survey response.
Álvarez Sauco M.: review and critique; evaluation of participants; survey response.
Sierra M.: review and critique; evaluation of participants; survey response.
Monje M. H. G.: review and critique; evaluation of participants; survey response.
Sánchez Ferro A.: review and critique; evaluation of participants.
Novo Ponte S.: review and critique; evaluation of participants; survey response.
Alonso-Frech F.: review and critique; evaluation of participants; survey response.
Macías-García D.: review and critique; evaluation of participants; survey response.
Legarda I.: review and critique; evaluation of participants; survey response.
Rojo A.: review and critique; evaluation of participants; survey response.
Álvarez Fernández I.: review and critique; evaluation of participants; survey response.
Buongiorno M. T.: review and critique; evaluation of participants; survey response.
Pastor P.: review and critique; evaluation of participants.
García Ruíz P.: review and critique; evaluation of participants; survey response.
Financial disclosuresSantos García D. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial and Teva.
López Ariztegui N. has received honoraria for educational presentations and advice service by Abbvie, Italfarmaco, Zambon, and Bial.
Cubo E: Travel grants: Abbvie, Allergan, Boston; Lecturing honoraria: Abbvie, International Parkinson's disease Movement Disorder Society.
Vinagre Aragón A. has received honoraria for educational presentations by UCB Pharma.
García-Ramos R. has received honoraria for educational presentations and advice service by Abbvie, Zambon, Merz, Allergan, Bial, Krka, Teva and Italfarmaco.
Borrué C.: None.
Fernández-Pajarín G. has received honoraria from Italfarmaco, Zambon and Bial and sponsorship from Italfarmaco, Boston Scientific and Zambon for attending conferences.
Caballol N. has received honoraria from Bial, Italfármaco, Qualigen, Zambon, UCB, Teva and KRKA and sponsorship from Zambon, TEVA and Abbvie for attending medical conferences.
Cabo I. has received honoraria for educational presentations and advice service by Abbvie, Zambon and Bial.
Barrios López J.M.: None.
Hernández Vara J. has received travel bursaries and educational grants from Abbvie and has received honoraria for educational presentations from Abbvie, Teva, Bial, Zambon, Italfarmaco and Sanofi-Genzyme.
Ávila Rivera M. A. has received honoraria from Zambon, UCB Pharma, Qualigen, Bial, and Teva, and sponsorship from Zambon and Teva for attending conferences.
Gasca-Salas C.: None.
Escalante S. has received honoraria for educational presentations and advice service by Abbvie, Zambon, and Bial.
Manrique de Lara P.: None.
Pérez Noguera R.:
Álvarez Sauco M. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Zambon, Bial and Teva.
Sierra M.
Monje M.H.G. has received speaker and travel honoraria from Novartis Pharmaceutical. She is employed by Fundación HM Hospitales. She is member of the Electronic Development of Rating Scales Committee of the Movement Disorders Society.
Sánchez Ferro A. has received funding from the Joint Program for Neurodegenerative Diseases (project Reference Number: HESOCARE-329-073) and has also received speaker and travel honoraria from Teva, Zambon, Abbvie, and Novartis Pharmaceutical. He is employed by Leuko Imaging Systems S.L. and Fundación HM Hospitales. He owns common stock in Leuko Labs, Inc a company with commercial interests in a Medical Device developed for neutropenia detection. He is also an inventor of a Method and Apparatus for Motor Function characterization (US 2020/0060622 Al) that has been licensed to an independent commercial entity (nQ-Medical) by the Massachusetts of Technology. He serves as Chair of the Ad Hoc Committee on Technology of the Spanish Neurological Society, Chair of Committee on Electronic Development of Rating Scales of the Movement Disorders Society and is a member of the Movement Disorders Society Technology Taskforce and Scientific and Education (European Section) Committee.
Novo Ponte S. has received unrestricted educational support from Abbvie and Zambon and honoraria for educational presentations from Abbvie and Italfarmaco.
Alonso-Frech F.: None.
Macías-García D. has received honoraria for educational presentations by Abbvie, Teva and Zambom.
Legarda I. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Zambon, Bial and Teva.
Rojo A. has received travel bursaries from Abbvie and Zambon and honoraria for educational presentations from Teva, Bial, UCB and Zambon.
Álvarez Fernández I.: None.
Buongiorno M. T.: None.
Pastor P: None.
García Ruíz P.
Conflict of interestsNone. This work has been carried out absolutely independently from the company sense4care, who has only provided the devices and has not participated in it.