Cerebrotendinous xanthomatosis (CTX) is an autosomal recessive disease caused by a deficiency of mitochondrial enzyme sterol 27-hydrolylase. Such a deficiency results in a reduced production of chenodeoxycholic acid and in an increased formation of cholestanol. It is clinically characterized by cataracts, diarrhoea, xanthomas, premature arteriosclerosis and a number of progressive neurological symptoms. Although cholestanol levels are used for the diagnosis of CTX, their correlation with the clinical symptoms and their prognostic usefulness have not been assessed so far.
MethodsWe reviewed 14 CTX patients diagnosed between 1995 and 2008 in two reference centres for the genetic diagnosis of this disorder, whose cholestanol levels had been recorded. We studied the main demographic, clinical and therapeutical data and their correlation with plasma cholestanol levels.
ResultsThe average cholestanol level at diagnosis was 105.8μmol/l. These levels did not correlate with any neurological symptoms or with disability at diagnosis scored by the EDSS. After treatment, all patients achieved a significant reduction in plasma cholestanol levels (average reduction of 91μmol/l in an average follow-up of 34 months), although only one patient remained clinically stable.
ConclusionsHigh cholestanol levels are very useful for diagnosis of CTX but they do not have a prognostic value (they do not correlate with severity). Normalization of cholestanol levels is not always associated with clinical stabilization. However, follow-up of cholestanol levels can be useful for the dose adjustment.
La xantomatosis cerebro-tendinosa (XCT) es una enfermedad autosómica recesiva producida por un déficit del enzima 27-hidroxilasa. Como consecuencia, existe una deficiencia de ácido quenodeoxicólico y una sobreproducción de colestanol que se deposita en los tejidos. Clínicamente cursa con cataratas, diarrea, xantomas y diferentes síntomas neurológicos. A pesar de que los niveles de colestanol se emplean en el diagnóstico de la XCT, se desconoce su correlación con la clínica y el pronóstico.
MétodosSe han revisado 14 pacientes de XCT, diagnosticados entre 1995 y 2008 en dos centros de referencia para el diagnóstico genético, en los que se había determinado el colestanol. Se han estudiado los principales datos demográficos, clínicos y terapéuticos y su posible relación con los niveles de colestanol.
ResultadosLa media de los niveles de colestanol al diagnóstico fue de 106μmol/l. No se encontró ninguna relación entre el colestanol plasmático y los diferentes síntomas neurológicos, ni con el grado de discapacidad al diagnóstico medido mediante la EDSS. Tras la instauración del tratamiento se obtuvo una reducción significativa del colestanol plasmático en todos los casos (reducción media de 91μmol/l en una media de 34 meses), a pesar de lo cual sólo un paciente se estabilizó clínicamente.
ConclusionesLa presencia de niveles elevados de colestanol es muy útil para el diagnóstico de la XCT, pero no tiene valor pronóstico (no se correlaciona con la situación funcional). Su normalización no siempre se acompaña de una estabilización clínica, pero su monitorización puede ser útil para el ajuste del tratamiento.
Cerebrotendinous xanthomatosis (CTX, OMIM # 213700) is a recessive autosomal disease produced by a deficit in the mitochondrial enzyme sterol 27-hydroxylase (CYP27A1)1 described by van Bogaert et al.2 in 1937.
From a clinical perspective, patients with CTX usually present a history of chronic diarrhoea since infancy.3 In the third decade of life, they have bilateral cataracts and tendinous xanthomas. Subsequently, neurological symptoms appear and these may be extremely varied: ataxia, paraparesis, cognitive impairment, psychiatric disorders, Parkinson's disease,4 polyneuropathy,5,6 epilepsy and others.1,3–5 Although tendinous xanthomas appear in the name of this illness, they are frequently absent, so this in no case excludes the diagnosis.7
The primary metabolic defect in CTX consists of an alteration in the synthesis of bile acids from cholesterol, due to mutations in the gene CY27A1, which encodes the mitochondrial enzyme sterol 27-hydroxylase.1,8,9 A deficiency in CYP27A1 leads particularly to a reduction in the synthesis of chenodeoxycholic acid (CDCA) and an increase in intermediate products, such as 7α-hydroxy-4-cholesten-3-one and its oxidation product, cholestanol. Cholestanol is also produced directly from the oxidation of cholesterol and is accumulated in all tissues, especially in the brain, lungs, the crystalline lens and the tendons of patients with CTX, so it is used as a biochemical marker for this entity.3 Low levels of cholestanol are present in most tissues of mammals and its absorption, along with that of phytosterols, is extremely low in humans.
However, an elevated level of cholestanol in plasma can be found in primary biliary cirrhosis and in cases of cholestasis10,11 as a consequence of the alteration in cholesterol homeostasis. On the other hand, cholesterol levels in plasma and the lipoprotein profile in CTX patients are usually normal or below the range (the lipoprotein profile is usually “anti-aterogenic”).3
Cranial magnetic resonance imaging (MRI) usually shows hyperintensity at the level of the dentate nuclei and the pyramidal tracts.12 Some authors have described the potential usefulness of functional tests, such as DATSCAN, to evaluate the impact of this disease on the central nervous system.13
Treatment with CDCA, which inhibits the anomalous synthesis of bile acid, is effective for correcting biochemical alterations14 and, according to some authors, it slows down the progression of the disease.3 This treatment is usually associated with hydroxymethylglutaryl-co-enzyme A (HMG-CoA) reductase inhibitors.3
Although cholestanol levels have been used in the diagnosis of this disease for many years, their possible prognostic value and usefulness for monitoring the course of the disease have not been greatly studied. We present here a retrospective analysis of Spanish patients with CTX to evaluate the usefulness of cholestanol levels in this illness.
Patients and methodsA total of 26 patients with CTX (from 19 families) were reviewed; they all had a confirmed molecular diagnosis at the Galician Public Foundation for Genomic Medicine in Santiago de Compostela and at the Ramón and Cajal Hospital in Madrid between 1995 and 2008.
Their main demographic details (gender, family origin, date of birth, age at the onset of symptoms, age at diagnosis, age at death) were all recorded, along with clinical information (cataracts, xanthomas, diarrhoea, pyramidalism, ataxia, Parkinson's disease, psychomotor delay, cognitive impairment, psychiatric alterations, polyneuropathy, epilepsy), complementary tests (cranial CT scan, cranial MRI, spinal MRI, genetic study, neurophysiological study), as well as the treatment received and their progress. Through the Expanded Disability Status Scale (EDSS) by Kurtzke15 estimated at diagnosis, it was possible to evaluate the degree of disability in these patients. As this study has been conducted by neurologists and systemic symptoms such as diarrhoea are affected by memory bias, the age of onset was established as of the date on which the first neurological symptoms were reported. In patients who started with symptoms in infancy (for example with backwardness at school) and could not remember an exact age, they were assigned 12 years old as the age of onset.
Of the 26 patients diagnosed, only 14 cases (5 males and 9 females) were selected for the present study; they were those from the 12 families in which a determination of cholestanol levels had been made at diagnosis. Of these 12 families, four came from the Galician area, four from Castilla y León, two from Andalusia, one for Extremadura and one from Castilla La Mancha. In only 5 cases (36%) was the existence of consanguinity identified.
The cholestanol determinations were mostly performed at the Clinical Biochemistry Institute at the “Hospital Clínico” in Barcelona. Cholestanol, together with the other plasma sterols, was isolated following alkaline hydrolysis and extraction with organic solvent, then analyzed using capillary gas chromatography in an Agilent 7890 chromatograph fitted with a flame detector, such as trimethylsilyl-derivative (BSTFA 1%/TMCS in pyridine Sigma). The column used was a DB1701 (S&W). Epi-coprostanol (Sigma) was used as the internal standard. The reference values for cholestanol (from 2 to 12.6μmol/L) correspond to individuals between 1 and 60 years of age not affected by any alteration in the metabolism of cholesterol, with levels of between 36 and 102μmol/L being diagnostic for CTX. In addition, most of the patients had had analyses done for total cholesterol, cholesterol fractions and triglycerides.
Due to the small sample size, the Mann–Whitney U test was used to compare cholestanol levels between the different clinical groups. Using Spearman's correlation, the cholestanol levels were compared on the basis of the different quantitative variables. Statistical significance was considered for p<0.05, always using bilateral significance. The SPSS programme (version 15.0 for Windows) (SPSS Inc) was used for the statistical analysis
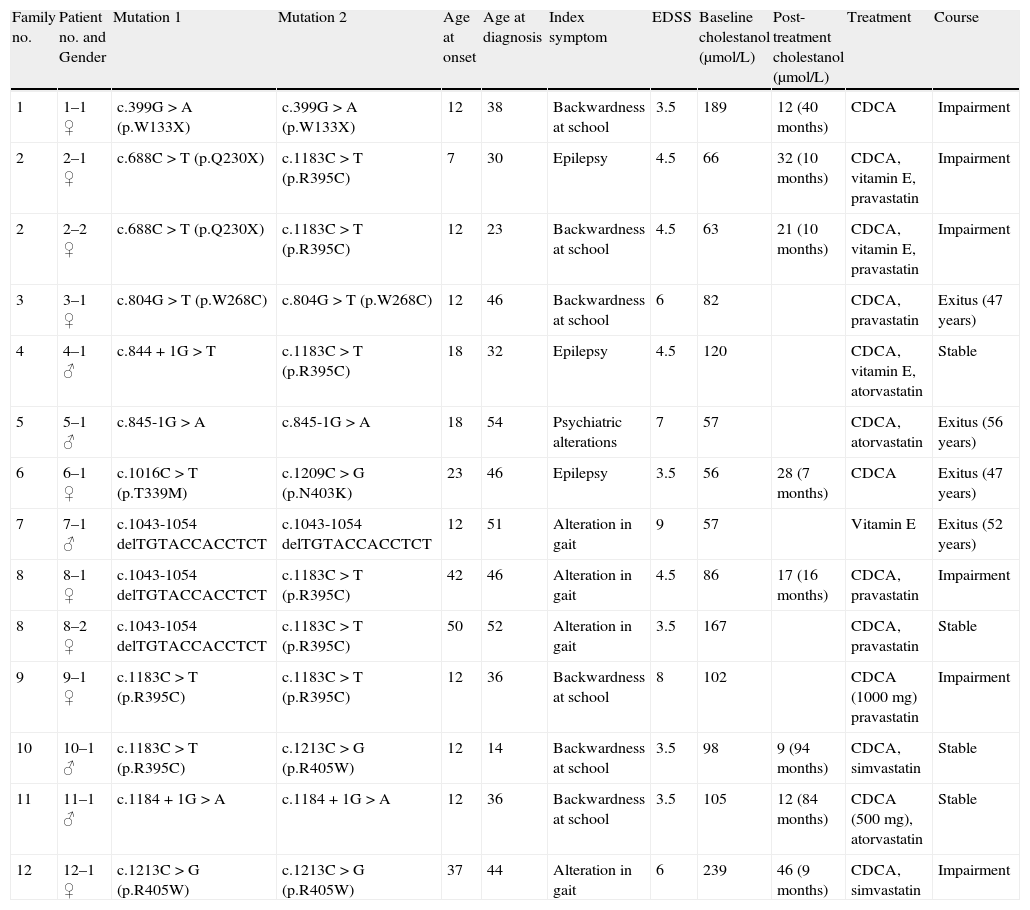
ResultsTable 1 shows details of the main demographic, clinical, genetic and biochemical data on the patients included in this series, together with their treatment and progression.
Main clinical, genetic and biochemical data for the 14 patients with CTX.
| Family no. | Patient no. and Gender | Mutation 1 | Mutation 2 | Age at onset | Age at diagnosis | Index symptom | EDSS | Baseline cholestanol (μmol/L) | Post-treatment cholestanol (μmol/L) | Treatment | Course |
| 1 | 1–1 ♀ | c.399G>A (p.W133X) | c.399G>A (p.W133X) | 12 | 38 | Backwardness at school | 3.5 | 189 | 12 (40 months) | CDCA | Impairment |
| 2 | 2–1 ♀ | c.688C>T (p.Q230X) | c.1183C>T (p.R395C) | 7 | 30 | Epilepsy | 4.5 | 66 | 32 (10 months) | CDCA, vitamin E, pravastatin | Impairment |
| 2 | 2–2 ♀ | c.688C>T (p.Q230X) | c.1183C>T (p.R395C) | 12 | 23 | Backwardness at school | 4.5 | 63 | 21 (10 months) | CDCA, vitamin E, pravastatin | Impairment |
| 3 | 3–1 ♀ | c.804G>T (p.W268C) | c.804G>T (p.W268C) | 12 | 46 | Backwardness at school | 6 | 82 | CDCA, pravastatin | Exitus (47 years) | |
| 4 | 4–1 ♂ | c.844+1G>T | c.1183C>T (p.R395C) | 18 | 32 | Epilepsy | 4.5 | 120 | CDCA, vitamin E, atorvastatin | Stable | |
| 5 | 5–1 ♂ | c.845-1G>A | c.845-1G>A | 18 | 54 | Psychiatric alterations | 7 | 57 | CDCA, atorvastatin | Exitus (56 years) | |
| 6 | 6–1 ♀ | c.1016C>T (p.T339M) | c.1209C>G (p.N403K) | 23 | 46 | Epilepsy | 3.5 | 56 | 28 (7 months) | CDCA | Exitus (47 years) |
| 7 | 7–1 ♂ | c.1043-1054 delTGTACCACCTCT | c.1043-1054 delTGTACCACCTCT | 12 | 51 | Alteration in gait | 9 | 57 | Vitamin E | Exitus (52 years) | |
| 8 | 8–1 ♀ | c.1043-1054 delTGTACCACCTCT | c.1183C>T (p.R395C) | 42 | 46 | Alteration in gait | 4.5 | 86 | 17 (16 months) | CDCA, pravastatin | Impairment |
| 8 | 8–2 ♀ | c.1043-1054 delTGTACCACCTCT | c.1183C>T (p.R395C) | 50 | 52 | Alteration in gait | 3.5 | 167 | CDCA, pravastatin | Stable | |
| 9 | 9–1 ♀ | c.1183C>T (p.R395C) | c.1183C>T (p.R395C) | 12 | 36 | Backwardness at school | 8 | 102 | CDCA (1000mg) pravastatin | Impairment | |
| 10 | 10–1 ♂ | c.1183C>T (p.R395C) | c.1213C>G (p.R405W) | 12 | 14 | Backwardness at school | 3.5 | 98 | 9 (94 months) | CDCA, simvastatin | Stable |
| 11 | 11–1 ♂ | c.1184+1G>A | c.1184+1G>A | 12 | 36 | Backwardness at school | 3.5 | 105 | 12 (84 months) | CDCA (500mg), atorvastatin | Stable |
| 12 | 12–1 ♀ | c.1213C>G (p.R405W) | c.1213C>G (p.R405W) | 37 | 44 | Alteration in gait | 6 | 239 | 46 (9 months) | CDCA, simvastatin | Impairment |
In the 14 patients with CTX, the mean age of onset of their neurological symptoms was 20 years (range from 7 to 50). The mean age at diagnosis was 39 years of age (range: 14–54), so the mean delay between onset of symptoms and their diagnosis was 19 years. Four patients had died in the period between their diagnosis and the performance of this study.
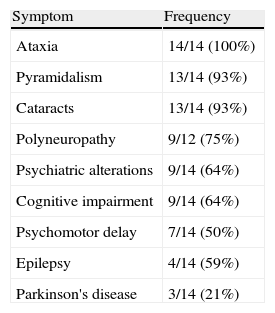
The index symptoms most frequently encountered were backwardness at school (43%), gait alterations (29%) and epileptic seizures (21%). Ataxia (100%), pyramidalism (93%) and cataracts (93%) were the most frequent symptoms (Table 2). Only 50% of the patients had xanthomas. The mean EDSS calculated on diagnosis was 5.1 (range: 3.5–9), representing severe disability.
The two sequencing variants considered as the pathogenic mutations in each case were identified in the 12 families. The most frequent mutation was p.R395C, which was found in up to 25% of the alleles (5 families).
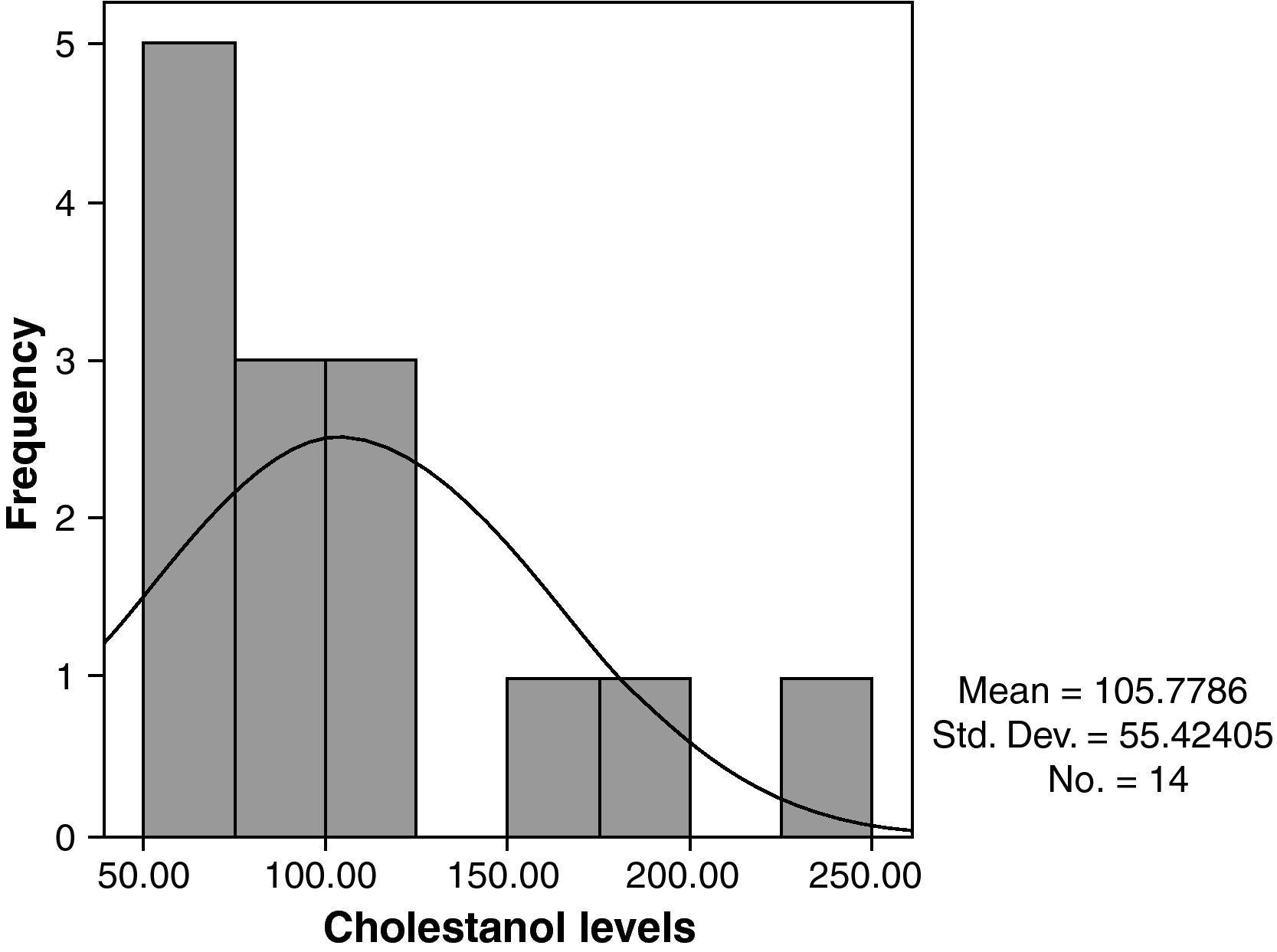
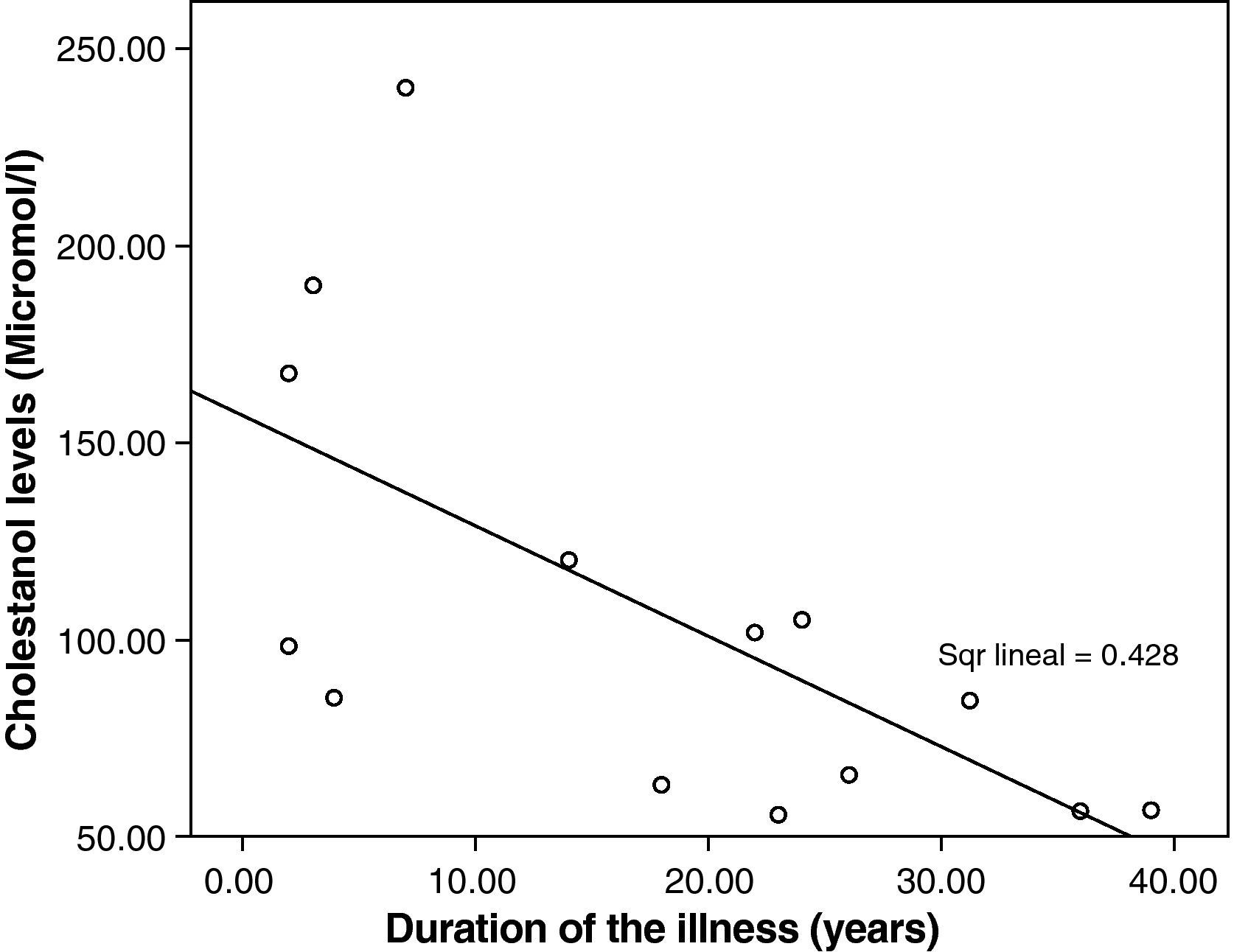
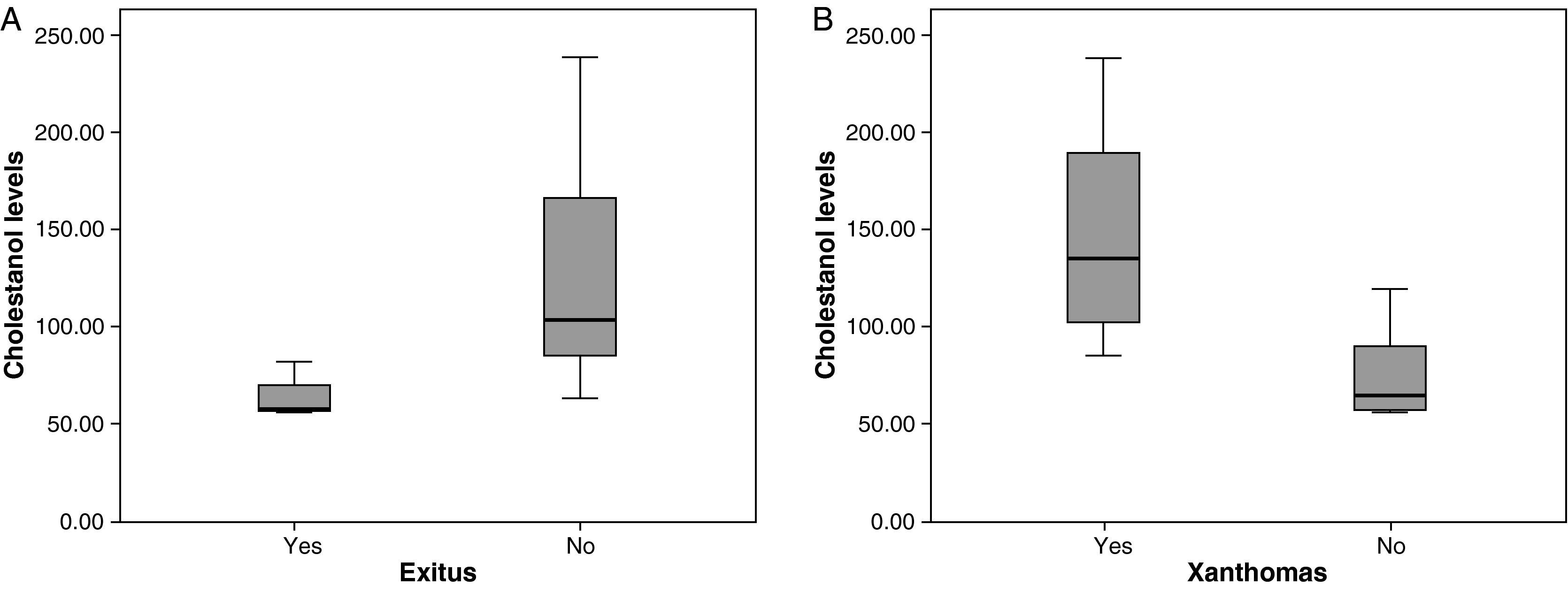
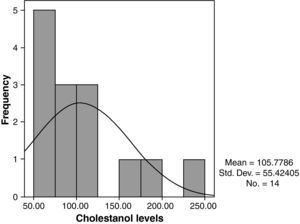
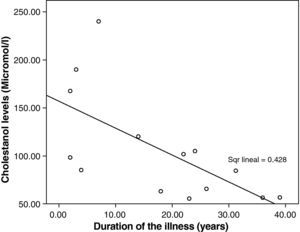
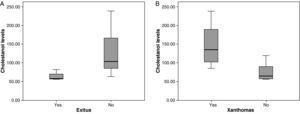
The mean cholestanol levels found were 106μmol/L (range: 56–238) (Fig. 1). A significant negative relationship (r=−0.64; p≤0.013) was found between the time during which people had suffered from the illness and their cholestanol levels, in other words cholestanol levels were lower the longer the illness had been present (Fig. 2). On the other hand, no significant relationship was found between plasma cholestanol and age at onset, at diagnosis or at death. Higher levels of cholestanol (116μmol/L, SD 65) were found among females than in men (87μmol/L, SD 28), although the small sample size meant that this difference did not achieve statistical significance. On the other hand, it is noteworthy that the cholestanol levels found in the group of patients who had died by the time of the study (63μmol/L; range: 56–82) were significantly lower than those obtained among those still alive at the same time (123μmol/L; range: 63–238) (p≤0.01) (Fig. 3A).
Higher levels of cholestanol (147μmol/L; SD 60) were found among the 6 patients who had tendinous xanthomas, versus 74μmol/L (SD 23) than among the 8 who did not (Fig. 3B) (p=0.01). Only one patient out of the 14 had no cataracts, and his levels of cholestanol were 57μmol/L, whereas the mean in the other 11 who did have cataracts was 109μmol/L (SE 56), although it did not achieve statistical significance.
With respect to neurological symptoms, it was only possible to relate cholestanol levels to the presence of polyneuropathy. Among the 3 patients without neuropathy, the levels found were 66μmol/L (SD 16), whereas the 9 with neuropathy had levels of 118μmol/L (SD 58) (p=0.052). In two cases, the review of the case history was not sufficient to confirm or rule out the presence of polyneuropathy. Although this difference did not achieve statistical significance, the presence of polyneuropathy can be seen as a trend in cases with higher serum levels of cholestanol. No differences were found in the levels on the basis of the types of neuropathy found in the electroneurogram. No statistically significant relationship was found between cholestanol levels and the degree of disability as measured through the EDSS at the moment of the diagnosis (p=0.3).
Nor was any statistically significant relationship found between cholestanol levels and the radiological findings (in both the cranial CT scan and in the cranial MRI, as well as in the spinal MRI), using evoked potentials and the electroencephalogram.
With respect to the lipid profile, this was normal in most cases. In only one patient, the triglyceride levels were a little high (175mg/dL; No.: 40–160mg/dL), with the mean triglyceride levels being 92mg/dL. Total cholesterol and LDL cholesterol were found to be normal in all cases, with means of 174mg/dL (No.: 120–220mg/dL) and 92.6mg/dL (No.: 60–190mg/dL), respectively. One patient presented low levels of HDL cholesterol (24mg/dL; No.: 30–100mg/dL), with mean HDL cholesterol levels of 59mg/dL. No correlation was found between the levels of cholestanol and levels of cholesterol, its fractions and triglycerides.
Of these 14 patients, one (7.1%) received only vitamin E (very late diagnosis; he died shortly after being diagnosed), two (14.3%) received only CDCA, 8 (57.17%) the association of CDCA and a statin, while three (21.4%) received the association of CDCA, a statin and vitamin E. All patients under treatment with CDCA received the standard dose (750mg), except for one patient who received 500mg (patient 11–1) and another who received 1000mg (patient 9–1).
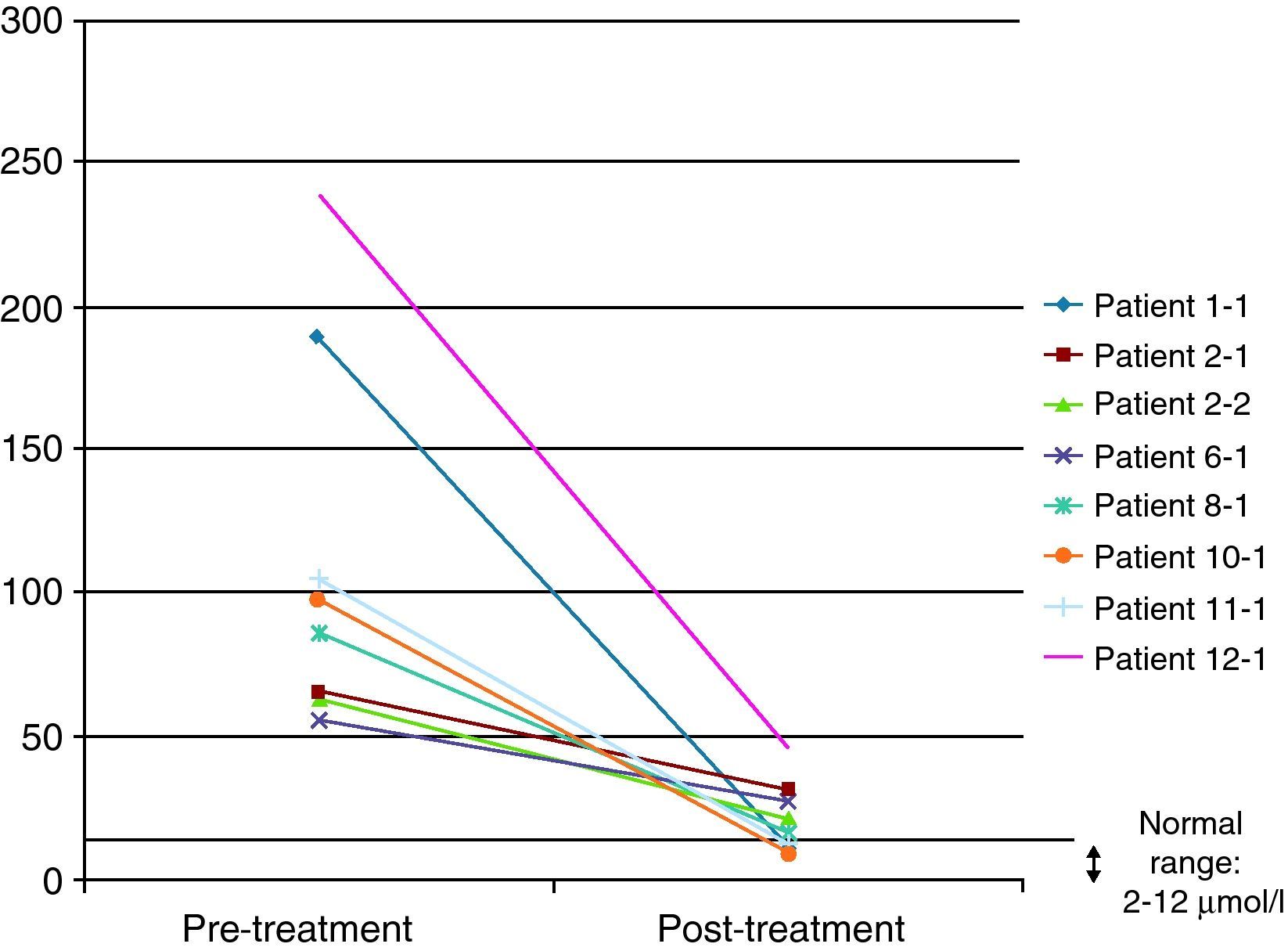
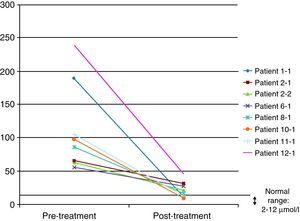
In 8 of the patients (57%), at least one further determination of cholestanol levels was made after the beginning of treatment in order to monitor their progression, with a significant reduction being observed in all cases and even a return to normal levels in three of them (37.5%). The mean reduction observed was 91μmol/L (range: 29–193) over a mean time of 34 months (range: 7–94) (Fig. 4). Despite the significant reduction in the cholestanol levels in the 8 patients monitored, only one of them (12.5%) became stabilized from a clinical standpoint.
Globally, despite treatment, only 4 of the 14 patients in the series (28.6%) became stabilized. In addition, 4 patients (28.6%) had died in the period between being diagnosed and the start of the present retrospective study, with a mean age at decease of 51 years (range: 47–56).
DiscussionThe goal of the present study was to analyze the possible relationship between the plasma levels of cholestanol and a range of clinical parameters and prognoses in CTX. For this purpose, we carried out a retrospective investigation through the review of the case histories of 14 Spanish patients with a genetic and biochemistry diagnosis of the disease.
Lipid profiles were normal in most patients. This coincides with what has been published previously in the medical literature, suggesting that the levels of cholesterol and its fractions are usually normal or a little low in patients with CTX.3,16
The mean cholestanol level found was 106μmol/L, so the levels in all patients with CTX studied were elevated (between 5 and 20 times the upper value of normality). Elevated cholestanol levels are not exclusive to CTX and can be observed, for example, in hepatic illnesses, phytosterolaemia, hypothyroidism and type II familial hyperlipoproteinaemia.11,17 Moderate elevations were also found in asymptomatic heterozygotic carriers, although never as low as the minimum levels detected in the patients with CTX.18,19
Higher levels of cholestanol were found among those patients with xanthomas, cataracts and polyneuropathy. These findings suggest the accumulation of cholestanol at the level of the tendons, crystalline lenses and the peripheral nervous system related to higher levels of cholestanol in circulation; that is to say, the accumulation of cholestanol in tissues would be favoured in patients with very elevated levels.
It is striking that no significant relationship was seen between cholestanol levels and the degree of disability as measured on the EDSS scale at the time of diagnosis. Although it is frequently taken for granted that higher levels of cholestanol are related to a worse functional prognosis, the results in our series do not support this interpretation. Nonetheless, the scale used present major limitations as it places excessive emphasis on motor symptoms versus the rest of the non-motor symptoms and, therefore, underestimates the complexity of the disease.
Some authors have used the monitoring of cholestanol levels for the therapeutic follow-up of patients.20 In the 8 patients in our series in whom at least one additional determination was effected after the start of therapy, the reduction was significant, with the levels even returning to normal in three of them. It is, therefore, striking that, despite presenting very elevated levels, it is possible to achieve a significant reduction with the same dose of CDCA (the standard dose was 750mg). Increasing CDCA doses in cases where levels do not return to normal is a moot point: the biochemical improvement did not correlate with a symptomatic improvement, as only one patient managed to stabilize from a clinical standpoint. This patient had been diagnosed at 14 years of age and is currently 22, so the main factor determining stabilization might have been the early institution of treatment.
Significantly lower levels of cholestanol were found in the group of patients who died in comparison with those still alive. Bearing in mind that the patients who died are those who were diagnosed with the greatest delay; this might indicate that, in the advanced stages of the illness, there is perhaps a fall in cholestanol levels. This agrees with the negative correlation found between the time since onset of the illness and plasma levels: patients with a longer history of illness presented lower levels of cholestanol.
Another possible explanation might be that the most severely affected patients are those with larger amounts of cholestanol deposited in the various tissues (crystalline lens, tendons, brain) and that is why they have lower levels of cholestanol circulating in their blood. Our data also show a poor correlation between plasma levels of cholestanol and functional prognosis. The reason why cholestanol is deposited selectively in certain tissues (in nerve tissue, for example, and more specifically in the dentate nuclei) and why some patients are more susceptible to this tissue accumulation, the cause of irreversible cerebral lesions (neuronal loss, accumulation of lipidic crystals) described in several autopsy-based studies12,21,22 is still unknown.
As a result, it is necessary to apply caution when interpreting the significance of plasma levels of cholestanol in patients with CTX. Elevated levels are very useful for establishing the diagnosis, but they have no prognostic value (i.e. higher levels are not necessarily related to a worse functional situation) and do not allow monitoring of the clinical effectiveness of treatment (normalization of levels is not always accompanied by clinical stabilization). On the other hand, they may be useful for adjusting CDCA dosage, as well as in the verification of therapeutic compliance.
The evaluation of our results presents several limitations. First, there is considerable data dispersion as each patient's cholestanol levels were determined at the moment of the diagnosis, corresponding in each case to a different moment in the natural course of the illness and at different ages. Second, we do not know the curve of the natural course of these levels throughout the course of the illness. Although our findings suggest an initial increase in terms of the start of symptoms, and subsequently a gradual decline as the disease develops, the transversal design of the study does not allow this hypothesis to be confirmed. Finally, the small sample size (due to the low prevalence of the disease) constrains the statistical analysis of the data. Thus, additional prospective studies, with more extensive series and detailed follow-up, are necessary to document more precisely the clinical value of cholestanol levels for the prognosis and monitoring of patients with CTX.
Conflict of interestThe authors have no conflict of interests to declare.
Our thanks to the neurologists who collaborated in the performance of this study: Dr. M. Arias (CHUS), Dr. A. Ares (Hospital de León), Dr. J. Duarte (Hospital General de Segovia), Dr. I. García Castañón (Hospital San Pedro Alcántara), Dr. A. López de Munain (Hospital de San Sebastián), Dr. A. Molina (Hospital 12 de Octubre), Dr. M. Moya (Hospital Línea de la Concepción), Dr. D. Ezpeleta (Hospital Gregorio Marañón) and Dr. F.J. Jiménez Jiménez (Hospital del Sureste).
Please cite this article as: Pilo de la Fuente B, et al. Utilidad de los niveles de colestanol en el diagnóstico y seguimiento de los pacientes con xantomatosis cerebrotendinosa. Neurología. 2011 26:397-404.
This paper was presented in part at the 13th Meeting of the European Federation of the Neurological Societies (Florence, September 12th-15th, 2009) as a poster entitled “Cerebrotendinous Xanthomatosis in Spain”. This work did not receive any funding.