Chorea or ballism is a hyperkinetic disorder characterised by involuntary, abrupt, irregular, large-amplitude movements due to basal ganglia lesions. Causes vary greatly, and include vascular, metabolic, degenerative, and infectious aetiologies; deficiency diseases, etc. Among the metabolic causes, chorea associated with hyperglycaemia is noteworthy: despite being an infrequent disease, it is potentially reversible when treated correctly.1
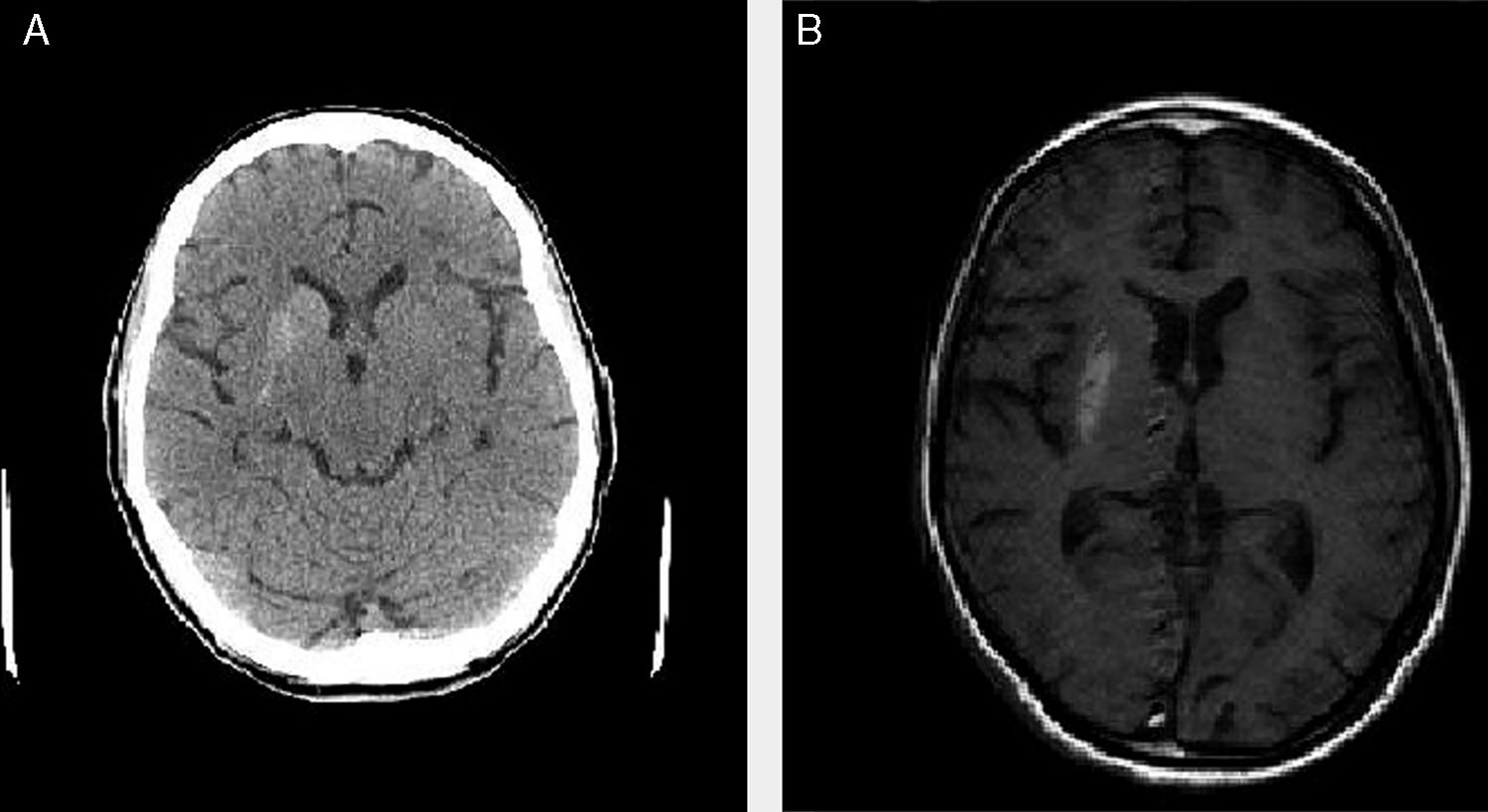
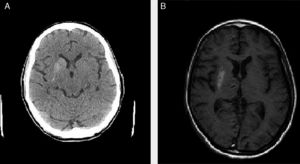
We present the cases of 4 patients (3 women and one man) who attended the emergency department due to uncontrollable choreic movements (predominantly left-sided in 3 and bilateral in one) progressing for more than 24hours (Table 1). All 4 cases were assessed by a neurologist in the emergency department. The rest of the neurological examination was normal. An emergency blood analysis including ions, urea, creatinine, glucose, complete blood count, coagulation, and venous gases revealed high glucose levels (ranging from 196 to 939mg/dL) and pH>7.3 in all cases. A urine analysis detected high glucose levels and absence of ketone bodies in 3 patients (trace values were detected in patient 2). Patients 1 and 3 had previously been diagnosed with diabetes mellitus, whereas patients 2 and 4 had not. All patients presented arterial hypertension and dyslipidaemia under treatment. All 4 cases were admitted for study. During admission, a blood test including liver and kidney function tests, lipid profile, ions, thyroid hormones, vitamin B12, folic acid, complete blood count, iron profile, and coagulation returned normal results (except in one case, showing known kidney failure, which was under follow-up); serology tests for HIV, HCV, HBV, and Treponema pallidum were negative in all cases. All patients showed glycated haemoglobin levels far above normal limits (13.8%-16.5%). All patients underwent emergency CT and MRI scans in the inpatients ward; the brain CT scan revealed (uni- or bilateral) basal ganglia hyperdensities that corresponded to hyperintensities on the T1-weighted MRI sequence, with no alterations in the T2-weighted sequence (Fig. 1). We started symptomatic treatment in all 4 cases (tetrabenazine plus benzodiazepine and/or haloperidol in patients 2, 3, and 4) and adjusted or started anti-diabetic treatment (insulin and/or oral anti-diabetic drugs), which clearly improved symptoms. At discharge, choreic movements had disappeared in 3 patients and were less intense and frequent in the remaining patient (patient 1).
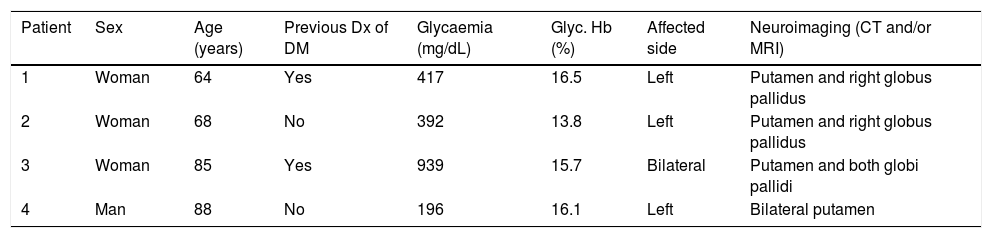
Clinical, laboratory, and radiological data of the 4 patients with chorea/ballism secondary to non-ketotic hypergycaemia.
| Patient | Sex | Age (years) | Previous Dx of DM | Glycaemia (mg/dL) | Glyc. Hb (%) | Affected side | Neuroimaging (CT and/or MRI) |
|---|---|---|---|---|---|---|---|
| 1 | Woman | 64 | Yes | 417 | 16.5 | Left | Putamen and right globus pallidus |
| 2 | Woman | 68 | No | 392 | 13.8 | Left | Putamen and right globus pallidus |
| 3 | Woman | 85 | Yes | 939 | 15.7 | Bilateral | Putamen and both globi pallidi |
| 4 | Man | 88 | No | 196 | 16.1 | Left | Bilateral putamen |
CT: computed tomography; DM: diabetes mellitus; Dx: diagnosis; Glyc. Hb: glycated haemoglobin; MRI: magnetic resonance imaging.
According to the literature, and as in the cases described, chorea or ballism secondary to hyperosmolar non-ketotic hyperglycaemia mainly affects women aged between 60 and 80 years.2 It usually manifests in patients diagnosed with type 2 diabetes mellitus with poor glycaemic control, although it has also been described in cases of type 1 diabetes mellitus and diabetes onset, as in cases 2 and 4.3 Diagnosis is based on laboratory and neuroimaging data and on a temporal association between treatment onset and clinical improvement. The pathophysiological mechanism remains unknown. The most widely accepted theory today is that the state of anoxia generated by hyperglycaemia activates other pathways for obtaining energy by decreasing GABA and acetylcholine concentration.4 This would not be case with ketotic decompensations since acetoacetic acid represents a means for obtaining GABA. However, this theory does not explain all the cases described to date, since the condition has been observed in patients with ketotic hyperglycaemia or even hypoglycaemia. Thus, other hypotheses on the pathogenesis of chorea have emerged, including dopamine hypersensitivity (predominantly in women) and vascular insufficiency secondary to hyperglycaemia.5–7
In short, although this is an infrequent condition, it is important to consider hyperglycaemia as a possible cause of chorea, since early management is essential to symptom resolution.
Please cite this article as: González-Pinto González T, Pérez Concha T, Losada Domingo JM, Moreno Estébanez A. Corea/balismo secundaria a hiperglucemia no cetósica: serie de 4 casos. Neurología. 2020;35:134–136.